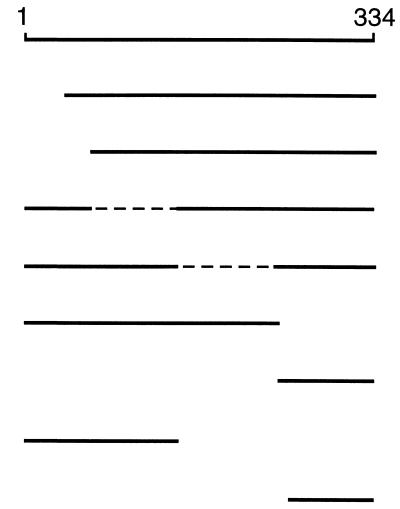
TABLE 3.
Effects of deletions in FliM on its function and binding to other proteinsa
a Summary of FliM deletions studied, their phenotypes, and the binding of FliM fragments to FliN, FliG, CheY, and phospho-CheY. Phenotypes of the deletions were determined by expressing the proteins in the fliM null strain DFB190. Binding was examined in coprecipitation experiments using the one-cell protocol and induction by 200 μM IPTG (interactions with FliN or FliG) or the two-cell protocol and induction by 100 μM IPTG (interactions with CheY or phospho-CheY). The symbols ++, +, +/−, and +/−− indicate four levels of binding, differing from each other by a factor of about 2 in the amount of coprecipitated FliM; ND, not determined; WT, wild type. Broken lines indicate internal deletions in FliM.