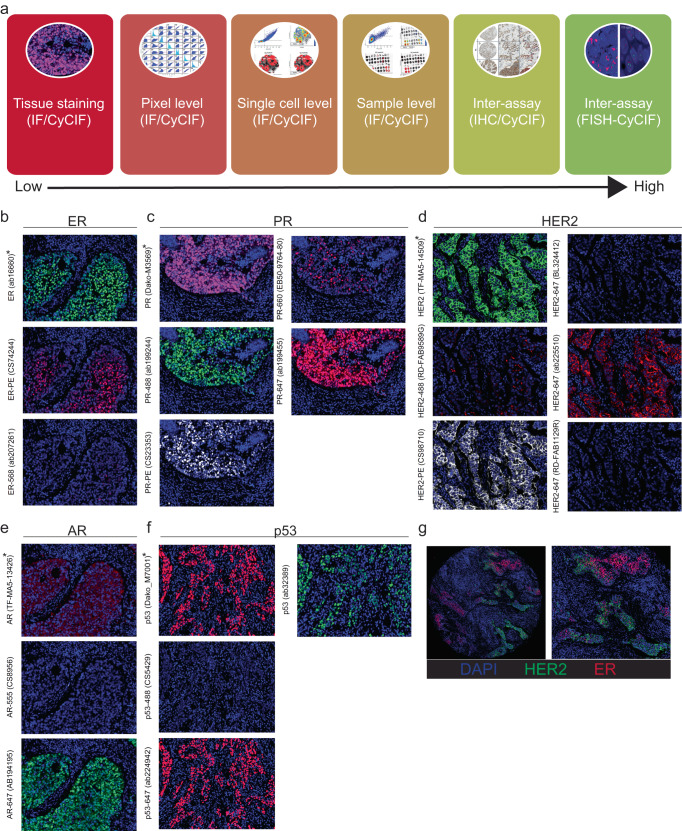
Fig. 1. Overview of fluorescent CyCIF antibody qualification against antibodies used in the clinical laboratory.
To qualify breast cancer-related antibodies HER2, ER, PR, AR, and p53, the BC03 tissue microarray (TMA), which represents 16 breast tumors in duplicate, was used. Multiple CyCIF antibodies were compared to a single antibody commonly used in clinical practice as a reference. a Schematic representation of the different levels of fluorescent antibody validation using the CyCIF method, starting from tissue staining (lowest level of validation) towards patient-level (highest level) inter-assay comparison (i.e., direct comparison of each patient tissue to itself between assays). b–f Representative CyCIF images of antibodies tested by CyCIF on the BC03 TMA. Asterisks indicate clinical antibodies (*) and qualified CyCIF antibodies (**) for each target. g Representative CyCIF image of HER2 (TF-MA5-14509; sp3) and ER (CS98710) staining, showing the majority of tumor cells are ER+, and some showing strong, membrane staining for HER2. Left image is a full TMA core (36× mag.); the right image corresponds to the left image (74× mag.).