Abstract
Breast cancers (BC) are rare in men and are often caused by constitutional predisposing factors. In women, mosaic BRCA1 promoter methylations (MBPM) are frequent events, detected in 4–8% of healthy subjects. This constitutional epimutation increases risk of early-onset and triple-negative BC. However, the role of MBPM in male BC predisposition has never been assessed. We screened 40 blood samples from men affected by BC, and performed extensive tumour analysis on MBPM-positive patients. We detected two patients carrying MBPM. Surprisingly, tumour analysis revealed that neither of these two male BCs were caused by the constitutional BRCA1 epimutations carried by the patients.
Keywords: HBOC syndrome, Breast neoplasm, Genetic testing, Homologous recombination, DNA methylation, Epigenomics
Highlights
-
•
Mosaic BRCA1 promoter methylations (MBPM) are frequent epimutations in women.
-
•
We report the first two male breast cancer patients carrying MBPM.
-
•
They presented invasive breast cancers expressing estrogen receptors.
-
•
Their breast cancers were not due to BRCA1: no homologous recombination deficiency.
-
•
Additional studies are necessary to use MBPM status to guide clinical decisions.
1. Introduction
Breast cancer (BC) is very rare in men, with less than five cases per million per year [1]. Genetic testing in affected males is recommended as approximately 10 % of cases are caused by germline pathogenic variants (gPV) in BRCA2, and another 2 % by gPV in BRCA1 [2]. In most cases however, no genetic cause is identified.
Constitutional methylation, where methylation of specific genes is detectable in normal tissues, is increasingly being considered as a mechanism for cancer predisposition. For instance, up to 1 % of Lynch syndrome cases could be due to constitutional MLH1 promoter methylation [3]. For now, constitutional epimutations cannot be used to adapt cancer prevention strategies, but they may help to guide BC screening in the future [4]. Indeed, mosaic BRCA1 promoter methylations (MBPM) are much more frequent than BRCA1 gPV in healthy women population (4–8% versus around 0.5 %), and several studies have shown that MBPM increase the risk of triple-negative or early-onset BC [[5], [6], [7], [8], [15]]. However, there is no consensus about the use of MBPM in clinical practice. Indeed, MBPM occur in utero in a subset of cells [[9], [15]]. Thus, the proportion of cells carrying BRCA1 methylation varies among tissues making follow-up guidelines difficult to establish. This variability has also led to heterogenous results in studies using different techniques to detect MBPM [4]. High-sensitivity techniques targeting core BRCA1 promoter, such as Methylation-Sensitive High-Resolution Melting (MS-HRM), are recommended [10,11]. Among remaining questions, involvement of MBPM in male BC has never been assessed. We describe here the first male BC patients carrying MBPM.
2. Patients and methods
We selected 40 men affected by invasive BC of no special type between 32 and 85 years old (Table 1). They did not carry germline pathogenic single nucleotide or copy number variant in 37 previously tested cancer-predisposing genes (Supplementary Table 1). All patients were born XY males and self-identified as men. According to French regulation, they had given informed written consent for genetic analysis for research purposes. We performed MS-HRM with EpiMelt BRCA1 kit (Methyldetect) on DNA extracted from blood samples of each patient.
Table 1.
Cohort of 40 men affected by breast cancer of no special type. ER: Estrogen Receptors; PR: Progesterone Receptors.
| Number (%) | ||
|---|---|---|
| Age at onset (years) | <40 | 1 (2.5) |
| 40–49 | 2 (5) | |
| 50–59 | 7 (17.5) | |
| 60–69 | 13 (32.5) | |
| 70–79 | 12 (30) | |
| >80 | 5 (12.5) | |
| Total | 40 (100) | |
| Immunochemistry | ER + | 39 (97.5) |
| PR + | 33 (82.5) | |
| Her2 amplification | 1 (2.5) | |
| Other cancers | Prostate | 6 (15) |
| Bladder | 1 (2.5) | |
| Seminoma | 1 (2.5) |
In MBPM-positive patients, we performed MS-HRM on DNA extracted from buccal swabs and fresh frozen tumour samples, as well as in available blood samples from affected relatives. Genomic tumour analysis consisted in targeted sequencing of more than 500 genes involved in cancer, detection of deletions causing loss of heterozygosity (LOH) at the BRCA1 locus, and a Shallow Whole Genome Sequencing to count “large-scale genomic alterations” (LGA), i.e. copy number variations larger than 10 Mega-bases. Counting LGA is an effective method for detecting homologous recombination deficiency (HRD) [8,12,13]. A tumour is considered HRD with a high level of certainty when it carries more than 20 LGAs.
3. Results
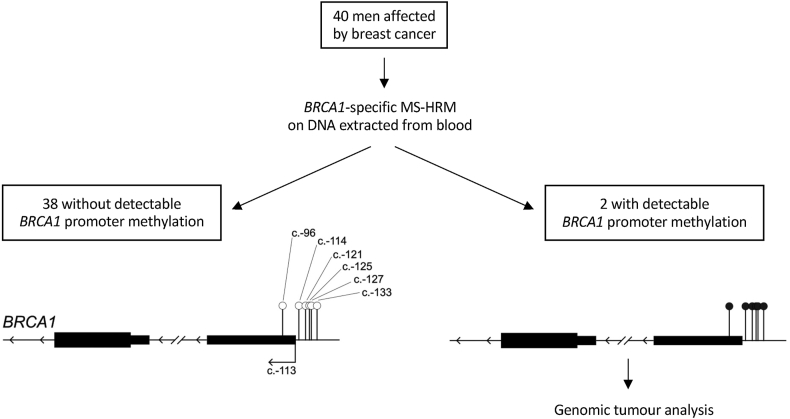
Two patients (5 %) carried MBPM (Fig. 1), a similar proportion as in the non-cancer female population, and much less than in a previous cohort of female family breast/ovarian cancer (44 %) [5,6,8].
Fig. 1.
Study design and graphic representation of BRCA1 (NM_007294) promoter analysis. BRCA1-specific Methylation-Sensitive High-Resolution Melting (MS-HRM) analyse 6 CpGs located in core BRCA1 promoter, nearby transcription start site. Unmethylated CpGs are represented by empty circles, methylated CpGs are represented by black-filled circles.
Clinically, Patient 1 was diagnosed at age 83 years with a tumour expressing estrogen receptors (ER) and progesterone receptors (PR), without Her2 overexpression. Treatments included mastectomy with lymphadenectomy (4 out of 19 lymph nodes invaded) followed by adjuvant chemotherapy and radiotherapy. He had no clinical recurrence after six years of hormonotherapy. No other personal or family history were noted.
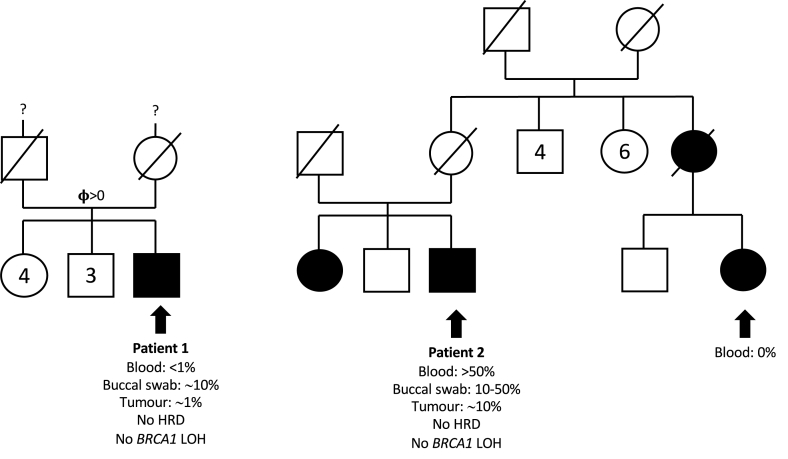
The medical history of Patient 2 included excess weight, diabetes, and arterial hypertension. His BC was diagnosed at age 69 years and expressed ER but not PR, without Her2 overexpression. He was treated by mastectomy with lymphadenectomy (0 of 6 lymph nodes invaded). He could not receive chemotherapy because of his comorbidities, but he underwent adjuvant radiotherapy and maintenance hormonotherapy. No clinical recurrence was noted four years after surgery. Several BCs were reported in his family (Fig. 2).
Fig. 2.
Pedigrees of the first reported men affected by breast cancer who carry mosaic BRCA1 promoter methylation. BRCA1 methylation levels are indicated. Breast cancer patients are shown in black. HRD: homologous recombination deficiency; LOH: loss of heterozygosity.
In Patient 1, MBPM was detected in <1 % of alleles in blood. As expected for a constitutional mosaic event, the proportion of methylated alleles was different in another non-cancerous tissue: ∼10 % in buccal mucosal smears. In Patient 2, ∼50 % of alleles were methylated in blood, and between 10 % and 50 % in buccal mucosal smears. His female cousin, affected with a bilateral BC at age 45 years and a third BC at age 63 years, was also analysed (Fig. 2). She had no gPV in 7 BC predisposing genes (BRCA1, BRCA2, PALB2, RAD51C, RAD51D, CDH1, TP53) and no MBPM detected in blood.
We then explored tumours from Patients 1 and 2. BRCA1 methylation could be detected in both patients, but at lower levels than in non-cancerous tissues: ∼1 % in Patient 1 and ∼10 % in Patient 2. These results suggested that tumours did not arise from cells carrying BRCA1 methylation, and that BRCA1 methylation was detected from adjacent non-cancerous tissue in those tumour samples. Tumour DNA sequencing showed no genetic variants of interest, and no LOH at the BRCA1 locus. Contrary to BC from female MBPM carriers, none of the two tumours presented an HRD signature, with only 3 and 9 LGAs in Patient 1 and Patient 2, respectively [8].
The reduced level of BRCA1 methylation in tumour samples compared to non-cancerous samples, the absence of BRCA1 LOH, and the absence of HRD signature were consistent with the absence of causality between MBPM and BC in these two patients.
4. Conclusions and discussion
Overall, this work found no evidence of the involvement of MBPM in male BC predisposition. These negative results appeared surprising regarding the role of MBPM in female BC predisposition. Male and female BCs are molecularly distinct diseases, but they share several predisposing genetic factors, such as gPV in BRCA1, BRCA2, PALB2 or CHEK2 [2]. In women, the relative risk of early-onset BC (before the age of 40 years) is up to 33 for BRCA1 gPV-carriers, while it is estimated only around 3.5 for MBPM-carriers [6,14]. Consequently, a significant fraction of BCs observed in MBPM-carrying women is not due to MBPM [7]. Here, we report the first two cases of MBPM in males affected by BC. Their BCs were not due to MBPM. Further studies are needed to clarify several pending questions. Larger cohorts could determine if MBPM is a rare predisposition factor that we could not detect in our small population. For instance, MBPM could increase risk of specific rare subtypes of male BC, such as triple-negative BC. Moreover, the role of constitutional epimutations in other genes, such as BRCA2 or PALB2, should be studied in male and female BC predisposition.
Ethics approval
All subjects provided written consent for the use of their samples for genetic studies and research purposes, according to French legislation.
CRediT authorship contribution statement
Mathias Schwartz: Writing - original draft, Validation, Methodology, Formal analysis, Data curation, Conceptualization. Sabrina Ibadioune: Validation, Methodology, Investigation, Formal analysis, Conceptualization. Sophie Vacher: Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Marie-Charlotte Villy: Writing - review & editing, Validation, Resources, Investigation, Data curation. Olfa Trabelsi-Grati: Validation, Investigation, Formal analysis. Jessica Le Gall: Validation, Investigation, Formal analysis. Sandrine M. Caputo: Writing - review & editing, Validation, Resources, Investigation, Data curation. Hélène Delhomelle: Validation, Resources, Investigation. Mathilde Warcoin: Validation, Resources, Investigation, Data curation. Virginie Moncoutier: Validation, Formal analysis, Data curation. Christine Bourneix: Validation, Investigation, Formal analysis. Nadia Boutry-Kryza: Writing - review & editing, Validation, Resources, Formal analysis. Antoine De Pauw: Validation, Resources, Formal analysis. Marc-Henri Stern: Writing - review & editing, Investigation, Conceptualization. Bruno Buecher: Writing - review & editing, Validation, Resources, Investigation. Emmanuelle Mouret-Fourme: Validation, Resources, Investigation, Data curation. Chrystelle Colas: Validation, Supervision, Resources, Investigation. Dominique Stoppa-Lyonnet: Writing - review & editing, Validation, Supervision, Resources, Project administration. Julien Masliah-Planchon: Writing - review & editing, Validation, Supervision, Project administration, Investigation, Formal analysis, Conceptualization. Lisa Golmard: Writing - review & editing, Validation, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Ivan Bieche: Writing - review & editing, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
Thank to A.D.T. International for proofreading the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2023.103620.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Miao H., Verkooijen H.M., Chia K.-S., Bouchardy C., Pukkala E., Larønningen S., et al. Incidence and outcome of male breast cancer: an international population-based Study. J Clin Orthod. 2011;29:4381–4386. doi: 10.1200/JCO.2011.36.8902. [DOI] [PubMed] [Google Scholar]
- 2.Campos F.A.B., Rouleau E., Torrezan G.T., Carraro D.M., Casali da Rocha J.C., Mantovani H.K., et al. Genetic landscape of male breast cancer. Cancers. 2021;13:3535. doi: 10.3390/cancers13143535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hitchins M.P., Alvarez R., Zhou L., Aguirre F., Dámaso E., Pineda M., et al. MLH1-methylated endometrial cancer under 60 years of age as the “sentinel” cancer in female carriers of high-risk constitutional MLH1 epimutation. Gynecol Oncol. 2023;171:129–140. doi: 10.1016/j.ygyno.2023.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong E.M., Southey M.C., Terry M.B. Integrating DNA methylation measures to improve clinical risk assessment: are we there yet? The case of BRCA1 methylation marks to improve clinical risk assessment of breast cancer. Br J Cancer. 2020;122:1133–1140. doi: 10.1038/s41416-019-0720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lønning P.E., Nikolaienko O., Pan K., Kurian A.W., Eikesdal H.P., Pettinger M., et al. Constitutional BRCA1 methylation and risk of incident triple-negative breast cancer and high-grade serous ovarian cancer. JAMA Oncol. 2022 doi: 10.1001/jamaoncol.2022.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong E.M., Southey M.C., Fox S.B., Brown M.A., Dowty J.G., Jenkins M.A., et al. Constitutional methylation of the BRCA1 promoter is specifically associated with BRCA1 mutation-associated pathology in early-onset breast cancer. Cancer Prev Res. 2011;4:23–33. doi: 10.1158/1940-6207.CAPR-10-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prajzendanc K., Domagała P., Hybiak J., Ryś J., Huzarski T., Szwiec M., et al. BRCA1 promoter methylation in peripheral blood is associated with the risk of triple‐negative breast cancer. Int J Cancer. 2020;146:1293–1298. doi: 10.1002/ijc.32655. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz M., Ibadioune S., Chansavang A., Vacher S., Caputo S.M., Delhomelle H., et al. Mosaic BRCA1 promoter methylation contribution in hereditary breast/ovarian cancer pedigrees. J Med Genet. 2023 doi: 10.1136/jmg-2023-109325. jmg-2023-109325. [DOI] [PubMed] [Google Scholar]
- 9.Lønning P.E., Berge E.O., Bjørnslett M., Minsaas L., Chrisanthar R., Høberg-Vetti H., et al. White blood cell BRCA1 promoter methylation status and ovarian cancer risk. Ann Intern Med. 2018;168:326. doi: 10.7326/M17-0101. [DOI] [PubMed] [Google Scholar]
- 10.Wojdacz T.K., Dobrovic A. Methylation-sensitive high resolution melting (MS-HRM): a new approach for sensitive and high-throughput assessment of methylation. Nucleic Acids Res. 2007;35:e41. doi: 10.1093/nar/gkm013. –e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machaj F., Sokolowska K.E., Borowski K., Retfiński S., Strapagiel D., Sobalska-Kwapis M., et al. Analytical sensitivity of a method is critical in detection of low-level BRCA1 constitutional epimutation. Sci Rep. 2023;13 doi: 10.1038/s41598-023-43276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brugge P. Homologous recombination deficiency derived from whole-genome sequencing predicts platinum response in triple-negative breast cancers. Nat Commun. 2023;14:1958. doi: 10.1038/s41467-023-37537-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eeckhoutte A., Houy A., Manié E., Reverdy M., Bièche I., Marangoni E., et al. ShallowHRD: detection of homologous recombination deficiency from shallow whole genome sequencing. Bioinformatics. 2020;36:3888–3889. doi: 10.1093/bioinformatics/btaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antoniou A., Pharoah P.D.P., Narod S., Risch H.A., Eyfjord J.E., Hopper J.L., et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolaienko O, Eikesdal HP, Ognedal E, Gilje B, Lundgren S, Blix ES, et al. Prenatal BRCA1 epimutations contribute significantly to triple-negative breast cancer development. Genome Med. 2023;15:104. doi: 10.1186/s13073-023-01262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.