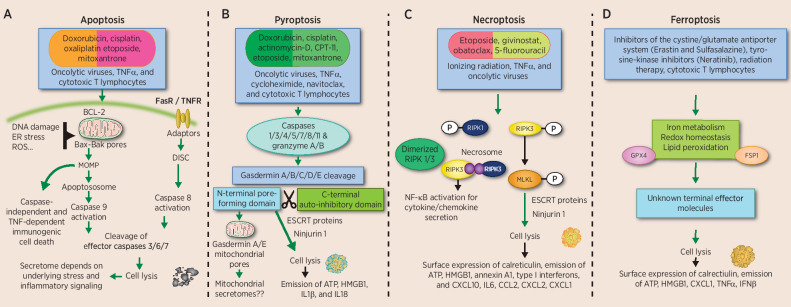
Figure 1.
Lethal stimuli and signaling pathways leading to activation of programmed cell death. Many cytotoxic anticancer treatments including chemotherapeutics, radiation, photodynamic therapy, biologics, and oncolytic viruses can trigger cell death. Apoptosis (A) can be initiated via the intrinsic or extrinsic pathway, both activating distinct signaling to emit secretomes that can differentially activate immunity against cancer. The role of pyroptosis (B) in anticancer immunity is accumulating. Activated caspases cleave gasdermin proteins to release the N-terminal domain that creates pores in the mitochondria (N-terminal gasdermin A and E) and the plasma membrane (all gasdermins, except Pejvakin). How the involvement of mitochondria and/or other organelles during pyroptosis affects immunogenicity remains unknown. Effector T cells and NK cells can also initiate tumor pyroptosis, although the role of this in further expanding T-cell responses needs to be investigated. Necroptosis (C) has been extensively studied in the context of cancer. The involvement of NFkB during necroptosis signaling contributes to inflammatory cytokines and chemokines that either result in antitumor or protumor outcome. In addition, MLKL overexpression can also initiate cell death and antitumor immunity. Ferroptosis (D) is an iron-dependent cell death involving lipid peroxidation. The immunogenicity of ferroptotic tumor cells is conflicting, although several immunostimulatory secretomes are released after this type of cell death. In addition, T cell–secreted IFNγ can trigger ferroptosis to potentiate the anticancer immunity cycle.