Figure 1.
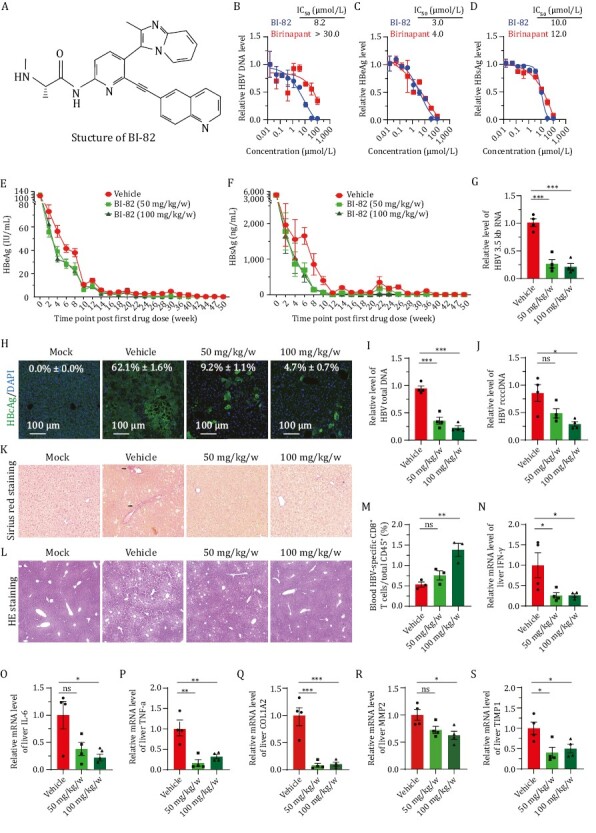
SMAC Mimetic BI-82 attenuates HBV infection in vitro and in vivo. (A) Structure of BI-82 and a cartoon scheme showing the mechanism of action of SMAC Mimetics. TNF-α interaction with TNFR1 and TNFR2 elicits either apoptosis by recruiting different adaptor proteins or NIK/TNF-α inflammation/survival pathway. The extrinsic pathway is triggered by binding of death receptors with death ligands (i.e., TNF-α, FasL, TRIAL, etc.) followed by formation of the death-inducing signaling complex, activation of caspase 8 and transmission of death signals to effector caspase, leading to apoptotic cell death. The intrinsic pathway is induced by a number of factors, including DNA damage to initiate the apoptotic cascade of death signals through interaction with specific downstream mediator of apoptosis. Then the formation of mitochondrial pores and change of membrane permeability release SMACs and cytochrome c into the cytoplasm to facilitate the activation of downstream apoptotic signal (caspase 9, 3, or 7), eventually resulting in apoptosis. Highly expressed IAPs inhibit apoptotic signaling cascades in tumor cells and infected cells with virus. SMAC Mimetics like BI-82, can specifically suppress IAPs protein levels to induce cell apoptosis. TNF, tumor necrosis factor; TRAIL, TNF related apoptosis inducing ligand; tBID, truncated BH3 interacting-domain death agonist; cIAP, cellular Inhibitors of apoptosis; XIAP, X-linked inhibitor of apoptosis. (B–D) Primary human hepatocytes (PHH) were treated with birinapant and BI-82, and infected with HBV for 6 days, then supernatants and cells were harvested to test viral titer through qRT-PCR for HBV DNA (B) and ELISA for HBs/eAg (C and D), n = 3. (E and F) BI-82 markedly inhibits HBeAg (E) and HBsAg (F) expression by ELISA in a persistent HBV mouse model. In 8–10 weeks old Alb-Cre male mice, 1.5 × 109 PFU of Ad-HBV was injected by intravenously administration. Next day, sera HBV antigens were quantified by ELISA for grouping infected mice with equal HBV titer. At 2 dpi, BI-82 were delivered by weekly oral administration for 16 weeks, n = 11. (G, I and J) BI-82 decreased intrahepatic RNA (G), HBV total DNA (I), and rcccDNA (J) level as measured by qRT-PCR at week 11 after administration. (H) BI-82 decreased intrahepatic HBcAg expression by immunofluorescence staining at week 11 after administration. (K) BI-82 protected liver from fibrosis induced by HBV as measured by Sirius red staining, black arrows indicate collagen fibers. (L) BI-82 protected the integrity of liver structure shown by HE staining. (M) BI-82 alleviated HBV-specific CD8+ T cell depletion. At Week 24, mice blood cells were stained with anti-mCD15, anti-mCD8 and anti-HBV-core tetramers, the HBV-specific CD8+ T cells were analyzed via FACS. (N–P) BI-82 attenuated HBV-induced intrahepatic inflammatory factors expression measured by qRT-PCR, including IFN-γ (N), IL-6 (O) and TNF-α (P). (Q–S) BI-82 attenuated HBV-induced intrahepatic fibrosis markers expression measured by qRT-PCR, including COL1A2 (Q), MMP2 (R), TIMP1 (S).
