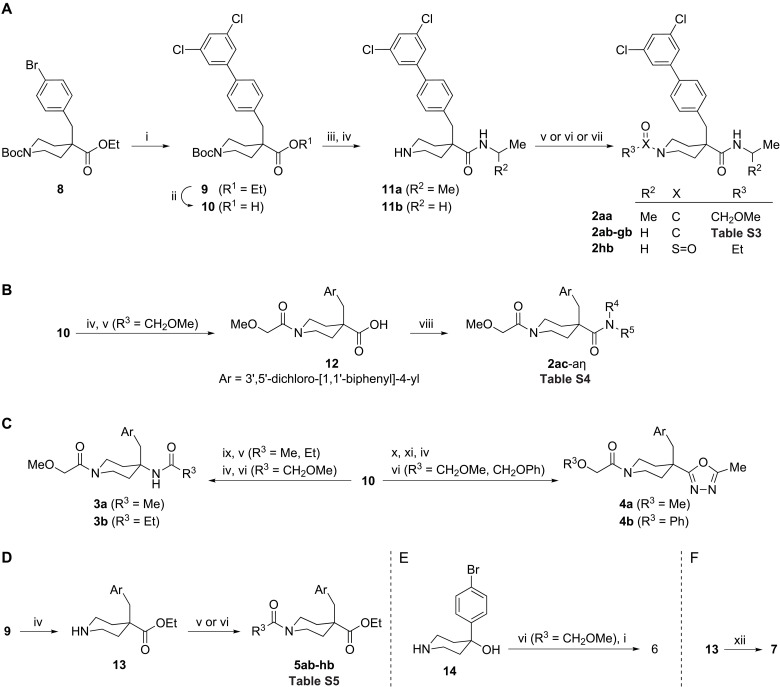
Fig. 3. Synthesis of isonipecotamide analogs 2 to 7 from precursors 8 and 14.
(A) Synthesis of 2aa to 2hb from 8. Biphenyl 9 and carboxylic acid 10 were obtained by cross-coupling followed by alkaline hydrolysis. Subsequent amidation and Boc-group removal yielded piperidine-4-carboxamides 11a to 11b. Piperidine acylation using suitable acid chlorides, carboxylic acids, as mediated by [Dimethylamino(triazolo[4,5-b]pyridin-3-yloxy)methylidene]-dimethylazanium hexafluorophosphate (HATU)], or ethanesulfonyl chloride yielded 2aa to 2hb. (B) Synthesis of 2ac to 2aη from 10. Piperidine 10 Boc removal followed by reaction with methoxyacetyl chloride gave isonipecotic acid 12, which was activated using HATU and coupled to a series of amines to yield isonipecotamides 2ac to 2aη. (C) Synthesis of 3 to 4 from 10. Conversion of acid 10 into a piperidin-4-amine followed by reaction with acid chlorides yielded amides that were subjected to Boc-group removal and acylation by methoxyacetic acid, as mediated by HATU, to yield 3a to 3b. The active ester of 10 was reacted with acethydrazide followed by in situ generated PPh3Cl2 resulting in an oxadiazole, which, after Boc removal and acylation by methoxy and phenoxyacetic acid, as mediated by HATU, gave 4a to 4b. (D) Synthesis of 5ab to 5hb from 9. Piperidine 9 Boc removal followed by piperidine acylation using acid chlorides or carboxylic acids, as mediated by HATU, yielded esters 5ab to 5hb. (E) Synthesis of 6 from 14. Piperidin-4-ol 14 was acylated by methoxyacetic acid, as mediated by HATU, and subjected to cross-coupling to yield biaryl 6. (F) Synthesis of 7 from 13. Reaction of piperidine 13, pentanal, and sodium cyanoborohydride gave amine 7. Reagents and conditions: (i) 3,5-Cl2C6H3B(OH)2, PdCl2(dppf), NaHCO3; (ii) LiOH, dioxane, H2O, 80°C, 12 to 24 hours; (iii) H2NCH(R2)Me, HATU, DIEA; (iv) TFA, DCM; (v) R3C(O)Cl, TEA (or DIEA), DCM; (vi) R3CO2H, HATU, DIEA, DMF (or MeCN); (vii) R3SO2Cl, TEA, DCM; (viii) HNR4R5, HATU, DIEA, DMF; (ix) DPPA, DIEA, PhMe, 100°C; (x) AcNHNH2, HATU, DIEA, MeCN; (xi) PPh3, C2Cl6, DIEA, MeCN; (xii) BuC(O)H, NaBH3CN, MeOH.