Abstract
Contradictory results are existed in the literature regarding the impact of trace elements on the pathogenesis of calcium oxalate (CaOx) stone patients. Therefore, the aim of our study was to investigate the effect of Cu and Zn on biochemical and molecular characteristics of CaOx stones. Plasma and urine concentrations of Cu and Zn in 30 CaOx stones patients and 20 controls were determined by flame atomic absorption spectrometry (FAAS). Urinary levels of citric acid and oxalate were measured by commercial spectrophotometric kits. Blood levels of glutathione reduced (GSH) and catalase (CAT) were determined as markers of antioxidant activity, while blood malondialdehyde (MDA) and urine level of nitric oxide (NO) were used to assess oxidative stress. Gene expression of MAPk pathway (ERK, P38, and JNK) were estimated. The plasma and urine levels of Cu were significantly increased in the patient group compared to those of controls, while the levels of Zn were decreased. Excessive urinary excretion of citric acid and oxalate were found among CaOx stone patients. The GSH and CAT concentration were significantly reduced in CaOx stones patients compared to healthy group. The plasma MDA and urine NO concentration were significantly increased in CaOx stones patients compared to control group. The expressions of the studied genes were significantly increased in CaOx stones patients. These findings suggest that alteration in Cu and Zn might contribute to pathogenesis of CaOx patients through oxidative stress and MAPK pathway genes (ERK, P38 and JNK).
Keywords: Calcium oxalate stones, Copper, Zinc, Oxidative stress, ERK, P38, JNK
Introduction
Kidney stone disease affects about 15–20% of the population and accounts for a major portion of global health-care costs [1]. If not treated appropriately, it might permanently damage the kidneys. The disease is more common in the Afro-Asian region [2].
One of the most prevalent components of kidney stones, calcium oxalate (CaOx), is implicated in the production of 80% of renal stones [3]. Damage to renal tubular epithelial cells and changes in crystal adhesion in renal tubular epithelial cells have been identified as essential factors in the production of CaOx stones [4, 5]. Environmental, genetic, and oxidative stress variables are thought to have a role in the disease's development. The fundamental process causing the shift in crystal adhesion in renal tubular epithelial cells, however, remains unknown.
Several studies have investigated the role of trace elements in the development of stone disease. However, the results of the studies on trace elements (such as copper (Cu) and Zinc (Zn)) are not consistent [6]. However, the significance of trace metals in the pathophysiology of kidney stones is unknown. According to certain research, dietary Zn consumption may be related with an increased risk of kidney stones, but manganese(Mn) intake may be associated with a lower risk of kidney stones [7]. Another study concluded that intake of Zn and iron was not related to a higher incidence of kidney stones. However, Cu intake may be correlated to an increased risk in some people [8]. Chi and colleagues explored that mineral concretions formed after inhibiting xanthine dehydrogenase were rich in Zn; additionally, inhibition of Zn transporter genes in the same model suppressed stone formation, implying that Zn may play a critical role in driving the process of heterogeneous nucleation [9]. According to the National Health and Nutrition Examination Survey, participants with a self-reported history of kidney stones had greater dietary Zn consumption [10]. A small case–control study, on the other hand, found reduced Zn intakes among individuals with stones [11]. Some investigations suggest that some major and trace elements have a role in the genesis of stone crystallization, either as a nucleus or nidus for the development of the stone or merely as an impurity of the stone structure [12].
P38 Mitogen-activated protein kinase (P38 MAPK) played a role in the development of atherosclerosis by influencing collagen expression directly [13]. Paleerath et al. found that the p38 MAPK signaling pathway was involved in the breakdown of tight junctions in epithelial cells, and that the expression of associated p38 MAPK signaling pathway proteins was up-regulated during CaOx stone formation [14]. The oxalate dramatically increased p38 MAPK activity considerably, increased c-Jun NH2-terminal kinase (JNK) pathway activity and phosphorylation slightly, and had no effect on extracellular signal-regulated kinase (ERK) pathway activity and phosphorylation [14].
Furthermore, CaOx crystals have been shown to enhance lipid peroxidation and free oxygen radicals, which mediate crystal development and adhesion to epithelial tubules, as well as renal tubule cell injury [15, 16]. Lipid peroxidation is oxidative tissue damage caused by oxygen free radicals and their biological products, such as superoxide, hydroxyl radicals, and hydrogen peroxide.
Although the fact that various studies have highlighted the potential roles of the above parameters and their significance in the development of stone disease, no clinical studies have linked changes in trace element levels (e.g., Cu and Zn) to the previously listed factors. The objective of the present study is to investigate the effect of Cu and Zn ions on biochemical and molecular characteristics of CaOx renal stones through a controlled clinical study.
Subjects and Methods
Subjects
This is a controlled clinical study that was carried out at the Mansoura Urology and Nephrology Center at Mansoura University after approval of the local ethical committee (MS.21.08.1605). The study included 30 consecutive patients with CaOx stones confirmed by Fourier transform infrared spectroscopy (FT-IR) and matched 20 healthy controls free of stone disease.
Collection of Urine and Blood Samples
All individuals were asked to provide 24-h urine samples. The patient was instructed to discard the first voided morning sample and start to collect the 24 h urine including the first voided urine in the morning of the following day. The urine samples were centrifuged at 3000 rpm for 10 min and analyzed for citrate, oxalate, nitric oxide (NO), Cu, and Zn levels. Blood samples were also collected from all individuals on tubes containing K2EDTA as an anticoagulant. A part of samples was used to detect the gene expression levels of the ERK, P38, and JNK genes. The other part was centrifuged at 4000 rpm for 10 min and the plasma was analyzed for Cu, Zn, reduced glutathione (GSH) concentration, catalase (CAT) activity, and malondialdehyde (MDA) concentration.
Methods
Determination of Cu and Zn Levels
Plasma and urine samples were digested as follows: 1.0 mL of plasma or 2.0 mL of urine samples, 3 mL HNO3, and 1 mL H2O2 were mixed in the digestion level and allowed to stand at room temperature for 15 min. The tubes were heated in a microwave oven (Speed wave four, Berghof Products, Germany) using a one-stage digestion program as follows: 1600 W (100%); 15-min ramp; 200 °C temperature; 15-min hold; and 15 min cooling [17]. After cooling, the solutions were diluted to 10 mL using deionized water then analyzed for Cu and Zn by using a Buck Scientific atomic absorption spectrometer (model 210 VCP, East Norwalk, CT, USA) equipped with air/acetylene flame and hollow cathode lamps for Cu and Zn at wavelengths of 324.8 and 213.9 nm, respectively and spectral bandwidth of 0.7 nm. Analysis of spiked samples was used to test the procedure accuracy, and the recovery rate was in the range of 97.5–99.0%. The precision did not exceed 3.0 percent (in terms of relative standard deviation).
Determination of Citric Acid and Oxalate in Urine
A 24-h urine sample was immediately centrifuged at 3000 rpm for 10 min at 4 °C. The supernatant was divided into aliquots and frozen at 80 °C in 1.5-mL tubes. Citric acid and oxalate were measured using the manual kits purchased from (Biochemical Enterprise, italy) [18].
Determination of Antioxidants (GSH, CAT) and Oxidative Stress (MDA, NO) Levels
The reduced glutathione (GSH) concentration, catalase (CAT) activity, and malondialdehyde (MDA) amount were detected in plasma samples by Bio-Diagnostic commercial kits (Giza, Egypt) according manufacturer [19]. Similarly, in urine samples nitric oxide (NO) concentration was measured [20]. A 7300 Genway spectrophotometer was used for all spectrophotometric measurements (Cole-Parmer Ltd., Staffordshire, UK).
Gene Expression Assay for ERK, P38, and JNK genes
QIAamp RNA Blood Mini Kit (supplied by QIAGEN Cat.NO.52304., USA) was used to extract the RNA from blood samples. The concentration and purity of RNA samples were determined by using a Thermo Scientific NanoDropspectrophotometer model 2000c (NanoDrop Technologies, Wilmington, idiumbromide(. The High Capacity cDNA reverse transcription Kit was used to convert RNA samples to complementary DNA (cDNA) (Thermo Fisher Scientific, Waltham, MA, USA(. cDNA samples were preserved at − 80 °C. SYPER Green PCR Master Mix was used for quantitative RT-PCR (Thermo Fisher Scientific, Waltham, MA, USA.(The mRNA expression levels of ERK, P38, JNK, and also GAPDH as a housekeeping gene (internal control) were measured using Step one plus real-time PCR (Applied Biosystems). The tests were carried out in triplicate. The primer sequences for the genes investigated are included in Table 1. The following programmer is used to adjust the PCR cycle parameters: pre-denaturation at 95 °C for 10 min, 40 cycles in denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min, and finally extension at 72 °C for 1 min.Using this equation RQ = 2−ΔΔCT, to calculate the relative quantification [17].
Table 1.
List of primer sequence
| Gene | Sequence | Product length(bp) | Accession no |
|---|---|---|---|
| P38 | F:5-GCATAATGGCCGAGCTGTTG -3 | 130 | NM_001315.3 |
| R:5-TCATGGCTTGGCATCCTGTT-3 | |||
| JNK | F:5-TTGGAACACCATGTCCTGAA-3 | 183 | NM_001278547.2 |
| R:5-ATGTACGGGTGTTGGAGAGC-3 | |||
| ERK | F: 5- ATCGCCGAAGCACCATTCAA-3 | 194 | NM_002745.5 |
| R: 5-AGGACCAGGGGTCAAGAACT-3 |
Statistical Investigation
Continuous variables were presented as mean ± SD and categorical variables as frequency and percentage. Student’s t-test and Chi square test were used as appropriate. The correlation between the continuous variables of both groups was calculated by Pearson coefficient correlation analysis with determination of r value. Results interpreted as strong correlation with r (0.7–1), moderate (0.3–0.7), weak (0.1–0.3), and no correlation (< 0.1). A software SPSS version 20 was used for statistical analysis of data (MAS Medical and Scientific Eq. Co, IL, USA), while Excel 2010 (Microsoft Office) was used for diagram production.
Results
Thirty patients with CaOx stones (15 males and 15 females) with a mean age of 51.4 ± 13.57 years were considered for the study. The controls consisted of 20 healthy individuals (10 males and 10 females) with a mean age of 53.2 ± 7.14 years. Both groups were matched in terms of age, gender, BMI, and diabetic status (Table 2).
Table 2.
Patients characteristics
| Item | CaOx patients | Control group | p value |
|---|---|---|---|
| Number | 30 | 20 | |
| Age (Years) mean ± SD | 51.4 ± 13.57 | 53.2 ± 7.14 | 0.596 |
| Gender (n, %) | |||
|
Male Female |
15(50) 15(50) |
10(50) 10(50) |
0.613 |
| BMI (kg/m2) mean ± SD | 33.6 ± 7.94 | 33.5 ± 6.24 | 0.914 |
| Diabetes, (n, %) | 6(20) | 6(30) | 0.506 |
|
Serum creatinine (mg dL−1) mean ± SD |
1.08 ± 0.46 | 0.93 ± 0.2 | 0.262 |
|
Uric acid (mg dL−1) mean ± SD |
6.29 ± 1.8 | 5.6 ± 1.14 | 0.145 |
Copper and Zinc levels in the Plasma and Urine
Table 3 shows plasma and urine Cu and Zn levels and Cu/Zn ratio in CaOx patients and control groups. CaOx patients have significantly higher plasma and urine Cu levels as well as a higher Cu/Zn ratio (p < 0.001) as compared to the control. Alternatively, levels of Zn in the plasma and urine in CaOx patients are considerably less than in the control group (p < 0.001).
Table 3.
Comparison between CaOx stone patients and control groups in the study parameters
| Marker | Ca oxalate patients | Control group | p value |
|---|---|---|---|
| Cu and Zn in blood and urine, mean ± SD | |||
| Cu(µg/L) in plasma | 114.2 ± 26.9 | 71.5 ± 16.9 | 0.001 |
| Cu(µg/L) in urine | 131.7 ± 34.1 | 73.6 ± 15.8 | 0.001 |
| Zn(µg/L) in plasma | 7.9 ± 2.8 | 13.6 ± 3.8 | 0.001 |
| Zn(µg/L) in urine | 5.8 ± 1.4 | 14.4 ± 3.8 | 0.001 |
| Cu/Zn ratio in urine | 23.8 ± 8.1 | 5.4 ± 1.5 | 0.001 |
| Cu/Zn ratio in plasma | 16.3 ± 7.5 | 5.6 ± 1.8 | 0.001 |
| Citric acid and oxalate in urine, mean ± SD | |||
| Citric acid (mg/24 h) | 513.1 ± 114.3 | 215.4 ± 50.1 | 0.001 |
| Oxalate(mg/24 h) | 19.2 ± 4.3 | 13.2 ± 2.6 | 0.001 |
| Antioxidant and oxidative stress markers in the blood, mean ± SD | |||
| GSH(mg/dl) | 6.1 ± 2.2 | 14.7 ± 3.6 | 0.001 |
| MDA(nmol/ml) | 8.5 ± 2.1 | 2.7 ± 0.9 | 0.001 |
| NO (µmol/L) | 9.5 ± 3.4 | 2.5 ± 0.81 | 0.001 |
| CAT (u/ml) | 0.6 ± 0.13 | 0.8 ± 0.13 | 0.001 |
| Gene expression by real time PCR (RT-PCR) in the blood, mean ± SD | |||
| ERK | 4.5 ± 1.3 | 1.03 ± 0.01 | 0.001 |
| P38 | 1.89 ± 0.58 | 0.94 ± 0.12 | 0.001 |
| JNK | 2.81 ± 0.63 | 1.01 ± 0.06 | 0.001 |
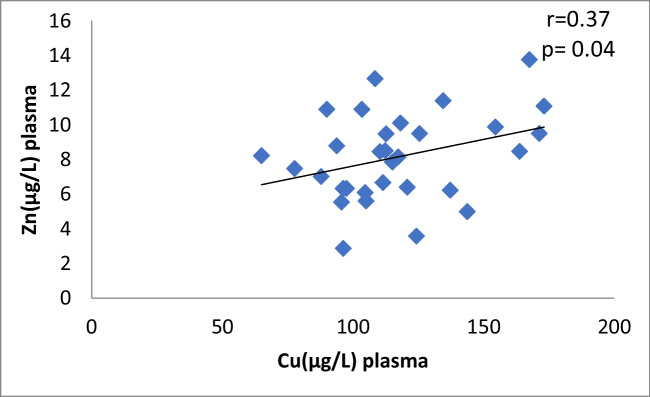
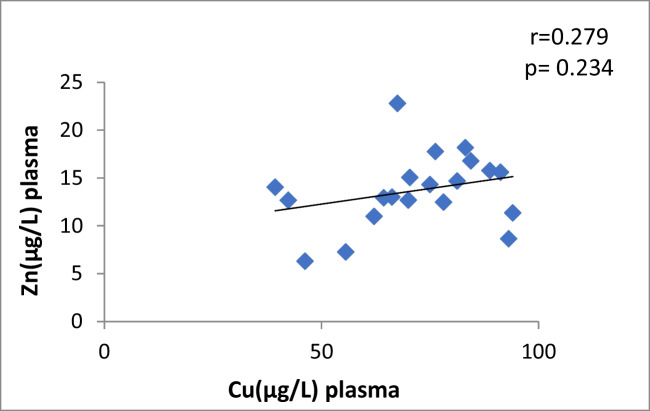
In patients with CaOx stones, there is a significant moderately positive correlation between Cu and Zn in the plasma (r = 0.37, p = 0.04) shown in Fig. 1, urine Cu and urine Zn showed no correlation (r = − 0.04, p = 0.82). Moreover, there is no correlation between plasma Cu and urine Cu (r = 0.09, p = 0.62) and no correlation between plasma Zn and urine Zn (r = 0.06, p = 0.73) (Table 4). The control group showed no significant correlation between Cu and Zn neither in plasma nor in urine (Table 4).
Fig. 1.
Scatter plot of plasma Cu and Zn in Caox patients
Table 4.
Pearson correlation Coefficients between different parameters in CaOx patients and control group
| The correlation | CaOx patients | Control group | ||||
|---|---|---|---|---|---|---|
| value of r | value of p | Interpretation | value of r | value of p | Interpretation | |
| Cu vs Zn in plasma | 0.37 | 0.04 | Moderate positive | 0.279 | 0.234 | No correlation |
| Cu vs Zn in urine | -0.04 | 0.82 | No correlation | -0.16 | 0.5 | No correlation |
| Cu in plasma vs urine | 0.09 | 0.62 | No correlation | -0.285 | 0.224 | No correlation |
| Zn in plasma vs urine | 0.06 | 0.73 | No correlation | -0.305 | 0.191 | No correlation |
| Cu in plasma vs CAT | 0.44 | 0.01 | Moderate positive | 0.149 | 0.532 | No correlation |
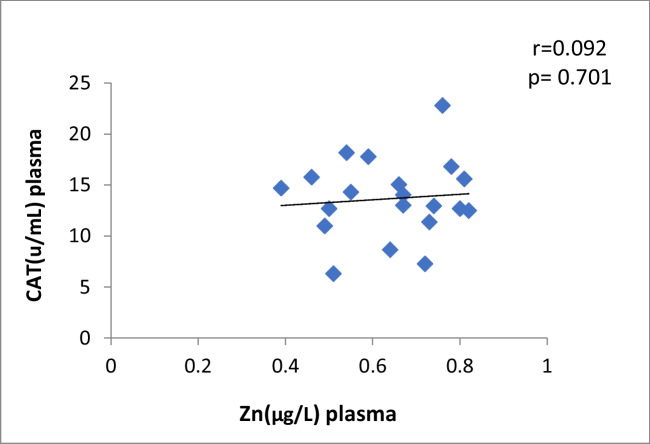
| Zn in plasma vs CAT | 0.37 | 0.04 | Moderate positive | 0.092 | 0.701 | No correlation |
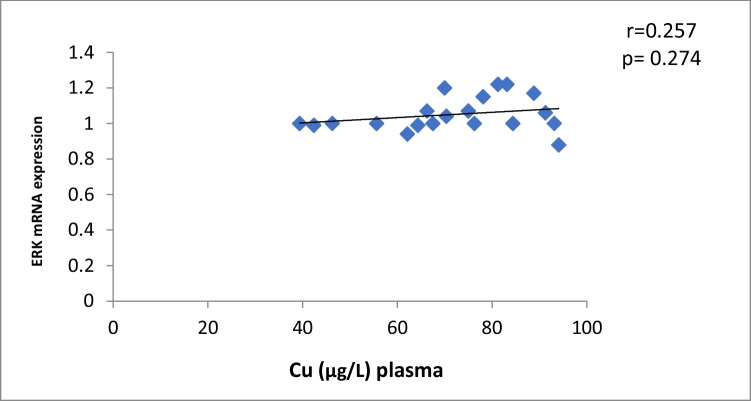
| Cu in plasma vs ERK | 0.38 | 0.03 | Moderate positive | 0.257 | 0.274 | No correlation |
| Cu in plasma vs P38 | -0.38 | 0.02 | Moderate negative | 0.207 | 0.382 | No correlation |
| Cu in plasma vs JNK | 0.37 | 0.04 | Moderate positive | -0.145 | 0.542 | No correlation |
| plasma Zn vs ERK | 0.13 | 0.47 | No correlation | 0.264 | 0.261 | No correlation |
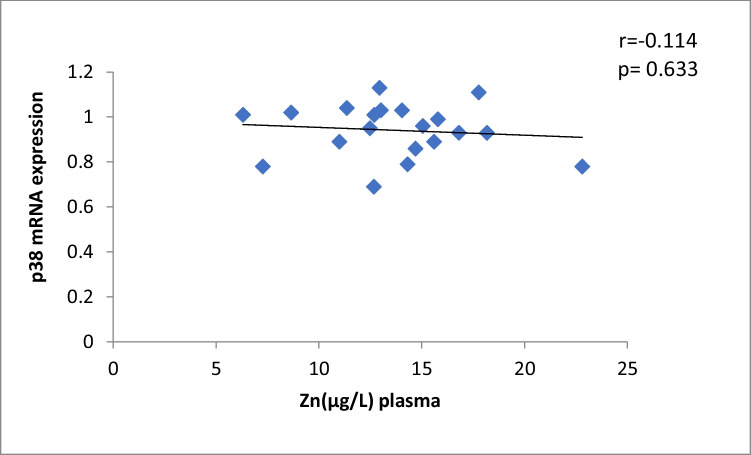
| plasma Zn vs P38 | -0.26 | 0.16 | No correlation | -0.114 | 0.633 | No correlation |
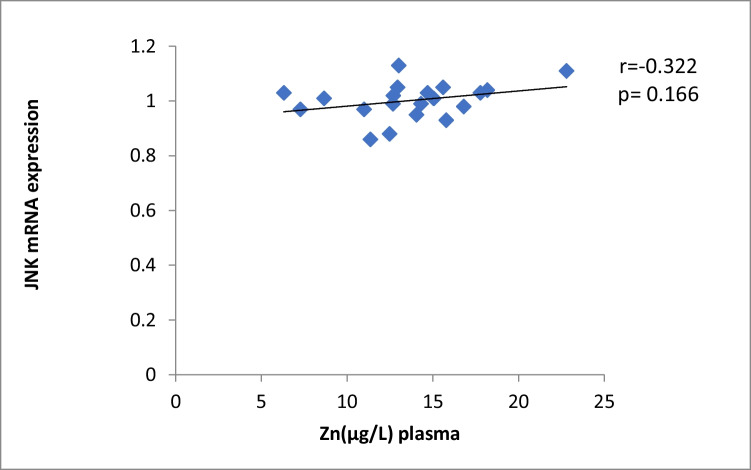
| plasma Zn vs JNK | 0.1 | 0.77 | No correlation | 0.322 | 0.166 | No correlation |
*Person test
Levels of Citric Acid and Oxalate in Urine
In comparison to the control group, urinary citric acid and oxalate concentrations were significantly higher in CaOx patients (p < 0.001) as presented shown in Table 3.
Antioxidants (GSH, CAT) and Oxidative Stress (MDA, NO) Levels
Table 3 shows that the GSH and CAT concentrations were significantly lower in patients with CaOx compared to the controls, while MDA and NO concentrations were higher in CaOx patients compared to controls (p < 0.001).
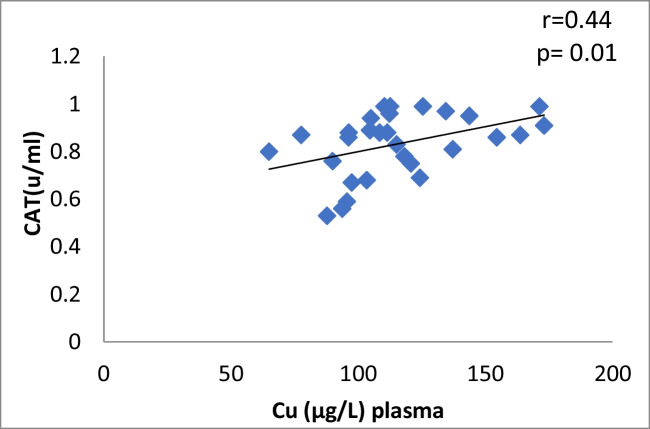
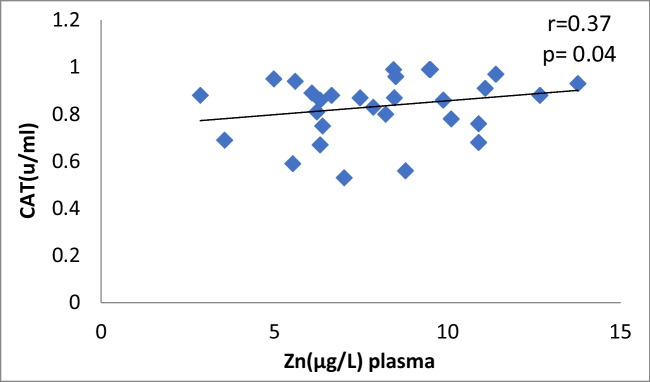
The correlation between plasma Cu and CAT levels in Ca oxalate patients showed a significant moderate positive (r = 0.44, p = 0.01) (Fig. 2). The data manifested a significant moderate positive correlation between plasma Zn and CAT activity in CaOx patients (r = 0.37, p = 0.04) (Table 4, Fig. 3). There is no correlation between antioxidants and oxidative stress markers and the levels of Cu and Zn in control subjects (Table 4).
Fig. 2.
Plasma Cu against CAT in Ca stone patients
Fig. 3.
Scatter plot of plasma Zn against CAT in Ca oxalate patients
Genetic Results of ERK, P38, and JNK by Real-Time PCR
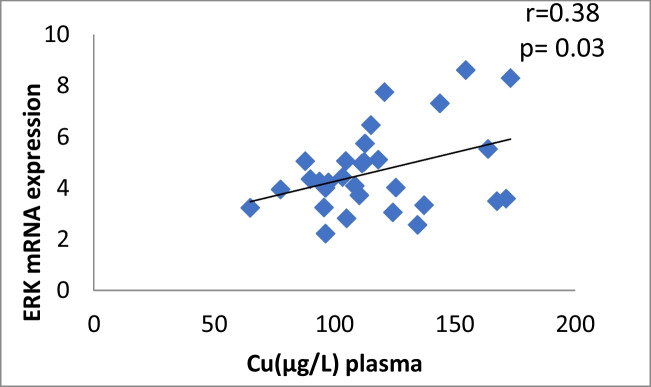
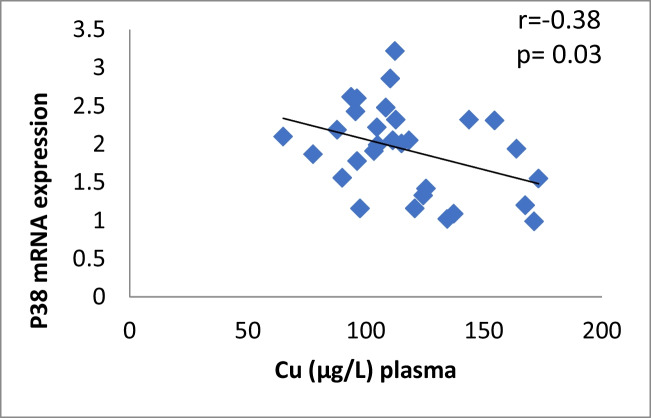
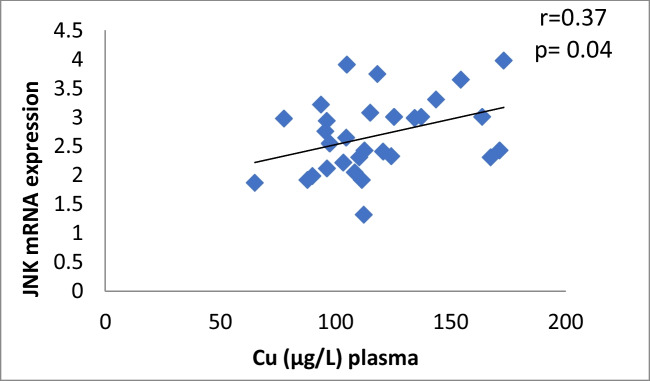
The results of our study (Table 3) indicated that the expression of ERK, P38 and JNK were considerably higher than in control group (p < 0.001). Cu levels in plasma revealed a moderately positive correlation with ERK and JNK mRNA expression (r = 0.38, 0.37; p = 0.03, 0.04, respectively). On the other hand, a moderately negative correlation was observed for P38 expression (r = -0.38, p = 0.02) (Table 4, Figs. 4, 5, 6). Zn levels in plasma showed no correlation with ERK, JNK and P38 mRNA expression (r = 0.13, 0.1; p = 0.47, 0.77; r = − 0.26; p = 0.16, respectively) (Table 4, Figs. 7, 8, 9, 10, 11, 12). There is no correlation between mRNAs expression levels and the levels of Cu and Zn in control subjects (Table 4, Figs. 13, 14, 15, 16, 17, 18).
Fig. 4.
Scatter plot of plasma Cu against ERK mRNA expression inCaOx patients
Fig. 5.
Scatter plot of plasma Cu against P38 mRNA expression in Ca stone patients
Fig. 6.
Scatter plot of plasma Cu against JNK mRNA expression in Ca oxalate patients
Fig. 7.
Scatter plot of plasma Zn against ERK mRNA expression in Ca oxalate patients
Fig. 8.
Scatter plot of plasma Zn against P38 mRNA expression in Ca oxalate patients
Fig. 9.
Scatter plot of plasma Zn against JNK mRNA expression in Ca oxalate patients
Fig. 10.
Scatter plot of plasma Cu and Zn in controls
Fig. 11.
Scatter plot of plasma Cu and CAT in controls
Fig. 12.
Scatter plot of plasma Zn and CAT in controls
Fig. 13.
Scatter plot of plasma Cu against ERK mRNA expression in controls
Fig. 14.
Scatter plot of plasma Cu against p38 mRNA expression in controls
Fig. 15.
Scatter plot of plasma Cu against JNK mRNA expression in controls
Fig. 16.
Scatter plot of plasma Zn against ERK mRNA expression in controls
Fig. 17.
Scatter plot of plasma Zn against p38 mRNA expression in controls
Fig. 18.
Scatter plot of plasma Zn against JNK mRNA expression in controls
Discussion
Controversial results are existing in the literature regarding the role of the trace elements on the CaOx stone pathogenesis. In the present study, we tried to shed the light on the effect of Cu and Zn on biochemical and molecular characteristics of CaOx stones in a controlled clinical study.
The current study showed that the urine citric acid content in CaOx patients was higher than in controls. This result is disagreeing with Huang et al. [21], who found that the urinary citric acid concentration in CaOx patients was significantly lower than in the healthy group. Our results regarding the low level of citrate in the study group is contradictory to the standard information that citrate is an inhibitor of crystallization. Therefore, it is supposed to show increase instead of decrease in the study group compared to controls. Nevertheless, this contradictory finding may be explained by the fact that the level of citric acid is influenced by the diet, medication, and lifestyle variations [22]. These co-variables together with the small sample size may explain this contradictory result. On the other hand, the level of urine oxalate was considerably higher in CaOx patients compared to the control group. This result is consisting with the results obtained by other researchers [21]. The high level of 24-h urinary oxalate causes an increase in the supersaturation of the CaOx solid phase, which leads to the production of CaOx stones [23].
Zn is considered as the second most common trace element in the human body. It has been described as an inhibitor of urinary stone development [6]. The Zn ions could chelate with oxalate ions, lowering oxalate activity and causing a decrease in nucleation rates [24]. In the present study, Zn concentrations in urine and plasma were shown to be considerably lower in CaOx stone patients as compared to control group. Our results are in line with Atakan et al. [6] who investigated the urine and plasma levels of Zn in CaOx stones patients and healthy individuals. They found that zinc was significantly lower in stone patients compared to healthy controls.
Cu enhances the crystallization of stone and affect calcium oxalate growth at very low concentrations, through forming insoluble salts with oxalate ions [12, 25]. Our results confirmed this theory as the level of Cu in urine was significantly increased in our patients compared to control group. This finding was reported by previous investigators [6]. The previous information supports our assumption that the increase in Cu and decrease of Zn may have a role in the pathogenesis of CaOx stones.
In this study, there was a significant increase in the Cu/Zn ratio in urine and plasma samples among CaOx stone patient compared to the control. The increase in Cu/Zn ratio in urine and plasma may contribute to the formation of CaOx stones. According to Khan et al. [26], Cu and Zn can deposit during the formation of stones between the surfaces of crystals of varying composition, resulting in laminations and more brittle lines in stones.
Numerous studies have shown that increasing the reactive oxygen species (ROS) and decreasing the antioxidants cause oxidative stress and contribute to the formation of kidney stones [27–29]. ROS are usually thought to be cytotoxic and can damage lipids, proteins, and DNA [30]. The present work showed that antioxidant markers (GSH and CAT) levels were significantly decreased in CaOx patients compared to control group, while ROS markers including MDA and NO were decreased. These results are confirmed by other investigators [21, 31, 32].
The current study revealed that the gene expression of ERK, P38 and JNK were significantly higher in CaOx patients compared with the control group; these results agree with other researchers [33]. Our results confirmed that MAPK signaling pathway has an essential role in the regulation of CaOx crystallization through, the increase of ROS generation and activation of the JNK, MAPK, and ERK signaling molecules [33].
In our study, the expression of p38, ERK, and JNK showed no correlation with Zn. Regarding the correlation of genes with Cu, ERK and JNK had positive correlation while P38 had negative correlation These observations are in agreement with other investigators [34]. The existence of these correlations in the study group and their absence in control group consolidate our assumption that the disturbance of these biochemical and molecular markers may have a role in the pathogenesis is in CaOx stones.
The present study is not free of limitations; one of these is the small sample size. So, studies with larger sample size are highly recommended to highlight the role of Cu and Zn and their correlations with MAPK signaling pathway in CaOx stones, which may help in understanding the pathogenesis of CaOx stones.
Conclusion
Changes in the level of Cu and Zn and the disturbance of each of antioxidants markers (CAT and GSH), ROS markers (MDA and NO), and MAPK pathway genes (ERK, P38, and JNK) may have an impact on the pathogenesis of CaOx.
Author Contribution
Shaimaa A.Y. Taha wrote the manuscript and performed experiments. Ahmed A. Shokeir designed the study and reviewed the manuscript. Wael I. Mortada analyzed data and revised the manuscript. Amira Awadalla performed experiments. Lamiaa A. A. Barakat designed the research and reviewed the manuscript. All authors approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). We would like to thank Ministry of Higher Education in Egypt for the financial support of this study.
Data Availability
All data and materials are available if requested.
Declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Informed consent was taken from all patients. Approval was granted by Local Institutional Review Board approval (MS.21.08.1605).
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ozturk H, et al. Protective effect of pentoxifylline on oxidative renal cell injury associated with renal crystal formation in a hyperoxaluric rat model. Urolithiasis. 2019;47(5):415–424. doi: 10.1007/s00240-018-1072-8. [DOI] [PubMed] [Google Scholar]
- 2.Chandrajith R, et al. Mineralogical, compositional and isotope characterization of human kidney stones (urolithiasis) in a Sri Lankan population. Environ Geochem Health. 2019;41(5):1881–1894. doi: 10.1007/s10653-018-0237-2. [DOI] [PubMed] [Google Scholar]
- 3.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327(16):1141–1152. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
- 4.Jonassen JA, et al. Oxalate-induced changes in the viability and growth of human renal epithelial cells. J Am Soc Nephrol. 1999;10(Suppl 14):S446–S451. [PubMed] [Google Scholar]
- 5.Khan SR. Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res. 1995;23(2):71–79. doi: 10.1007/BF00307936. [DOI] [PubMed] [Google Scholar]
- 6.Atakan IH, et al. Serum, urinary and stone zinc, iron, magnesium and copper levels in idiopathic calcium oxalate stone patients. Int Urol Nephrol. 2007;39(2):351–356. doi: 10.1007/s11255-006-9050-4. [DOI] [PubMed] [Google Scholar]
- 7.Ferraro PM, et al. Intake of trace metals and the risk of incident kidney stones. J Urol. 2018;199(6):1534–1539. doi: 10.1016/j.juro.2018.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu W et al (2022) Dietary copper intake and the prevalence of kidney stones among adult in the United States: A propensity score matching study. Front Public Health 10:973887 [DOI] [PMC free article] [PubMed]
- 9.Chi T, et al. A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease. PLoS ONE. 2015;10(5):e0124150. doi: 10.1371/journal.pone.0124150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J, McFann K, Chonchol M. Dietary zinc intake and kidney stone formation: evaluation of NHANES III. Am J Nephrol. 2012;36(6):549–553. doi: 10.1159/000345550. [DOI] [PubMed] [Google Scholar]
- 11.Tasian GE, et al. Dietary zinc and incident calcium kidney stones in adolescence. J Urol. 2017;197(5):1342–1348. doi: 10.1016/j.juro.2016.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh VK, Rai PK. Kidney stone analysis techniques and the role of major and trace elements on their pathogenesis: a review. Biophys Rev. 2014;6(3–4):291–310. doi: 10.1007/s12551-014-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Ma G, Chen X. Lipopolysaccharide induced LOX-1 expression via TLR4/MyD88/ROS activated p38MAPK-NF-κB pathway. J Vasc Pharmacol. 2014;63(3):162–172. doi: 10.1016/j.vph.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Peerapen P, Thongboonkerd V. p38 MAPK mediates calcium oxalate crystal-induced tight junction disruption in distal renal tubular epithelial cells. Sci Rep. 2013;3(1):1041. doi: 10.1038/srep01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thamilselvan S, Menon M. Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int. 2005;96(1):117–126. doi: 10.1111/j.1464-410X.2005.05579.x. [DOI] [PubMed] [Google Scholar]
- 16.Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol. 1997;157(3):1059–1063. doi: 10.1016/S0022-5347(01)65141-3. [DOI] [PubMed] [Google Scholar]
- 17.Mortada WI, et al. Copper and zinc levels in plasma and cancerous tissues and their relation with expression of VEGF and HIF-1 in the pathogenesis of muscle invasive urothelial bladder cancer: a case-controlled clinical study. J Environ Sci Pollut Res. 2020;27(13):15835–15841. doi: 10.1007/s11356-020-08113-8. [DOI] [PubMed] [Google Scholar]
- 18.Burtis CA, Ashwood ER, Bruns DE (2012) Tietz textbook of clinical chemistry and molecular diagnosis (5th edition). Journal of Clinical Biochemists of India 28(1):104–105
- 19.Hassanin MM, et al. Wogonin hampers dexamethasone-induced oxidative imbalance in sprague dawely rats. J Arch Pharm Sci Ain Shams Univ. 2020;4(1):70–78. [Google Scholar]
- 20.Ali A-F, et al. Oxidative state markers and clinicopathological findings associated with bovine leukemia virus infection in cattle. J Microbial Pathog. 2019;136:103662. doi: 10.1016/j.micpath.2019.103662. [DOI] [PubMed] [Google Scholar]
- 21.Huang HS, et al. Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology. 2003;62(6):1123–1128. doi: 10.1016/S0090-4295(03)00764-7. [DOI] [PubMed] [Google Scholar]
- 22.Vale L, et al. Metabolic evaluation in urolithiasis–study of the prevalence of metabolic abnormalities in a tertiary centre. Cent Eur J Urol. 2020;73(1):55. doi: 10.5173/ceju.2020.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bushinsky DA, et al. Calcium oxalate stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2002;61(3):975–987. doi: 10.1046/j.1523-1755.2002.00190.x. [DOI] [PubMed] [Google Scholar]
- 24.Barker T, Boon M, Jones FJJOCG. The role of zinc ions in calcium oxalate monohydrate crystallization. J Cryst Growth. 2020;546:125777. doi: 10.1016/j.jcrysgro.2020.125777. [DOI] [Google Scholar]
- 25.Tian Y, et al. Preliminary data on geochemical characteristics of major and trace elements in typical biominerals: from the perspective of human kidney stones. 2021;11(12):1396. [Google Scholar]
- 26.Khan SR, Hackett RL. Finlayson BJTJOU, Morphology of urinary stone particles resulting from ESWL treatment. J Urol. 1986;136(6):1367–1372. doi: 10.1016/S0022-5347(17)45340-7. [DOI] [PubMed] [Google Scholar]
- 27.Peng Z, et al. Inhalation of hydrogen gas ameliorates glyoxylate-induced calcium oxalate deposition and renal oxidative stress in mice. Int J Clin Exp Pathol. 2015;8(3):2680. [PMC free article] [PubMed] [Google Scholar]
- 28.Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33(5):349–357. doi: 10.1007/s00240-005-0492-4. [DOI] [PubMed] [Google Scholar]
- 29.Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol. 2013;189(3):803–811. doi: 10.1016/j.juro.2012.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linnane AW, Kios M, Vitetta L. Healthy aging: regulation of the metabolome by cellular redox modulation and prooxidant signaling systems: the essential roles of superoxide anion and hydrogen peroxide. Biogerontology. 2007;8(5):445–467. doi: 10.1007/s10522-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 31.Mulati M et al (2017) Effect of Klotho protein on renal oxidative stress in rat with calcium oxalate nephrolithiasis. Chinese J Urol 38(12):941–945
- 32.Thamilselvan V, Menon M, Thamilselvan S. mp20-14 uncoupled endothelial nitric oxide synthase contributes to oxalate-induced oxidative cell injury in renal epithelial cells: effect of antioxidants. J Urol. 2014;191(4S):e201–e201. doi: 10.1016/j.juro.2014.02.736. [DOI] [Google Scholar]
- 33.Yu L, et al. Calcium oxalate crystals induces tight junction disruption in distal renal tubular epithelial cells by activating ROS/Akt/p38 MAPK signaling pathway. Ren Fail. 2017;39(1):440–451. doi: 10.1080/0886022X.2017.1305968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C-C, et al. Involvement of oxidative stress-induced ERK/JNK activation in the Cu2+/pyrrolidine dithiocarbamate complex-triggered mitochondria-regulated apoptosis in pancreatic β-cells. J Toxicol lett. 2012;208(3):275–285. doi: 10.1016/j.toxlet.2011.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available if requested.