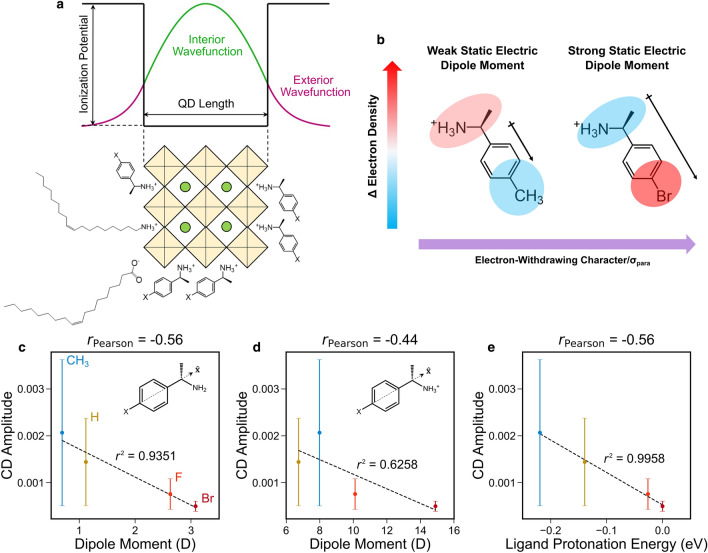
Figure 4.
Relationships between observed CD amplitude and overlap of the wavefunctions of the QD exciton and chiral ligand. In strongly confined QDs (such as 2 nm CsPbBr3 nanocrystals), there is appreciable tunneling of the exciton wavefunction outside the boundary of the nanocrystal, which may overlap with those of adsorbed ligands (a). Changing the para-position functional group modulates the electron density distribution across the ligand (b); electron-donating functional groups (e.g., CH3) increase electron density nearest the tethering group at which the ligand attaches to the QD surface, thereby enhancing the potential for wavefunction hybridization, while electron-withdrawing groups (e.g., Br) have the opposite effect. Ligand characteristics related to the movement of electron density away from the tethering group—the x-component (along the axis of opposing groups across the phenyl ring) of static electric dipole moment of neutral (R/S)-4-X-PEA (c) or protonated (R/S)-4-X-PEA+ (d), and the protonation energy of the amine (e)—are strongly inversely correlated with the excitonic CD of the corresponding ligand. Note that the Pearson correlation coefficients in (c–e) refer to the entire dataset, accounting for variation within sample groups, while the r2 values are calculated with respect to the means of each group (indicated by the circular symbols). Error bars represent the standard deviations of each group. The ligand protonation energy is referenced to the X = Br case.