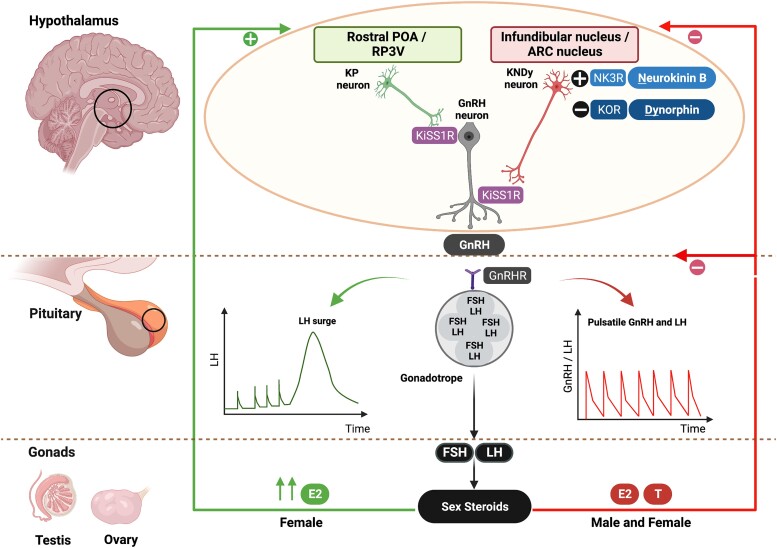
Figure 1.
KP and NKB in the regulation of the HPG axis. KP is released from the POA (equivalent to rostral periventricular area of the third ventricle, RP3V, in nonhumans) and infundibular nucleus (arcuate, ARC, nucleus in nonhumans) of the hypothalamus. The KP neurons in the infundibular nucleus coexpress NKB and dynorphin (known as KNDy neurons) and are involved in the autosynaptic regulation of pulsatile KP secretion via the NKB receptor (NK3R) and kappa opioid peptide receptor (KOR), respectively. Dynorphin inhibits, whereas NKB stimulates, KP release. Following KP's release from the hypothalamus, KP stimulates the hypothalamic GnRH neurons to release GnRH in a pulsatile manner, which stimulates anterior pituitary production of gonadotropins (LH, FSH) and subsequent production of gonadal (testicular/ovarian) sex-steroids (E2, T). The gonadotropins’ effect on the ovary stimulates follicular development, oocyte maturation, and ovulation. The KNDy neurons in the infundibular nucleus mainly receive negative feedback (E2, T) from sex-steroids, whereas KP neurons in the POA receive positive feedback from estrogen in females (high E2), which is involved in the preovulatory LH surge. Sex-steroid communication with the POA has not yet been fully established in males. E2, estrogen; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; HPG, hypothalamic-pituitary-gonadal; KISS1R, kisspeptin receptor; KOR, kappa opioid peptide receptor; KP, kisspeptin; LH, luteinizing hormone; NK3R, neurokinin 3 receptor; NKB, neurokinin B; P, progesterone; POA, preoptic area; RP3V, rostral periventricular area of the third ventricle; T, testosterone. Figure created with BioRender.com.