Abstract
Background:
Comprising nearly 35% of brain lipids, polyunsaturated fatty acids (PUFA) are essential for optimal brain function. However, the role of PUFA on cognitive health outcomes later in life is largely unknown.
Objective:
We investigated prospective associations of plasma phospholipid omega-3(ALA[18:3], EPA[20:5], DPA[22:5], DHA[22:6]) and omega-6(LA[18:2], AA[20:4]) PUFA with cognitive decline, risk of cognitive impairment and dementia among adults aged ≥65y in the Cardiovascular Health Study..
Methods:
Circulating fatty acid concentrations were measured serially at baseline(1992/1993), 6y and 13y later. Cognitive decline and impairment were assessed using the 100-point Modified Mini-Mental State Examination (3MSE) up to 7 times. Clinical dementia was identified using adjudicated neuropsychological tests, and ICD-9 codes.
Results:
Among 3,564 older adults free of stroke and dementia at baseline, cognitive function declined annually by approximately −0.5 3MSE points; 507 participants developed cognitive impairment and 499 dementia over up to 23y of follow-up. In multivariable models, higher circulating arachidonic acid(AA) concentrations were associated with slower cognitive decline and lower dementia risk, with associations growing stronger with greater length of follow-up (hazard ratio[HR,95% CI] of dementia per interquintile range, 0.74[0.56–0.97] at 5y, and 0.53[0.37–0.77] at 15y). Circulating docosapentaenoic (DPA) concentrations were associated with slower cognitive decline and lower risk of cognitive impairment (extreme-quintile HR, 0.72[95% CI: 0.55, 0.95]). Findings were generally null or inconsistent for other omega-3 or omega-6 PUFA.
Conclusion:
Circulating AA and DPA, but not other PUFA, are associated with slower rate of cognitive decline and lower risk of dementia or cognitive impairment later in life.
Introduction
Polyunsaturated fatty acids (PUFA) appear to be central to normal brain function and cognitive health[1]. For example, omega-3 alpha-linolenic acid (ALA), mostly derived from plant foods, may ameliorate oxidative stress, inflammation and apoptosis in neuron cells [2, 3]. Eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid, found in fish, can affect brain processes such as enhancing synaptic transmission and membrane fluidity [4]. Among dietary omega-6 PUFA, linoleic acid (LA), present in many plant oils, improves blood lipids and glucose-insulin homeostasis [5, 6],while sufficient levels of omega-6 arachidonic acid (AA), one of the most abundant fatty acids in the brain, are essential for neuronal growth, protection and repair [1]. Despite these plausible biological mechanisms, the role of PUFA on cognitive outcomes such as cognitive decline and dementia is largely unknown, particularly later in life. Notably, higher cellular membrane levels of PUFA has been identified among healthy centenarians[7], supporting a possible role of these fatty acids on membrane integrity and fluidity that could relate to healthy cognitive aging.
To elucidate the potential role of omega-3 and omega-6 fatty PUFA in cognitive health later in life, we investigated prospective associations of plasma phospholipid concentrations of ALA, EPA, docosapentaenoic acid (DPA), DHA, LA, and AA, each measured serially in the same participants at three points in time over 13 years, with incidence of dementia and cognitive decline in the Cardiovascular Health Study (CHS). The use of serial fatty acid measurements allowed estimation of long-term exposure to PUFA, which may be most relevant to cognitive health later in life.
Methods
In an ongoing population-based, prospective cohort study established by the National Heart, Lung, & Blood Institute, CHS investigates cardiovascular disease (CVD) risk factors among older US adults [8]. CHS enrolled 5,888 adults aged 65 years and older from four U.S. communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania) during 1989–1990 (5,201 participants) and 1992–1993 (687 participants)[8]. Annual clinic visits were conducted through 1999 to obtain repeated health assessments by collecting physical and cognitive measures, blood samples, and questionnaire data on demographics, health status, hospital stays, medical history, and lifestyle. Follow-up was performed via telephone interview in 2000 and twice yearly thereafter to collect information on health status and hospitalizations. Study procedures were performed by trained personnel using standardized procedures.
Fatty acid measurements
In total, 3,941 CHS participants (71% of living participants) had plasma phospholipid fatty acid concentrations measured in stored plasma specimens collected in 1992–93 (N=1,400 in 1998–99 (33%), and 935 in 2005–06 (41%). For the present investigation, we included 3,564 participants with circulating FA measures who were free of stroke or dementia in 1992–93, which serves as the baseline for this analysis (Supplemental Figure 1). Demographic characteristics, lifestyle and prevalence of comorbidities were similar when comparing participants included in this analysis to those excluded (flowchart shown in Supplemental Table 1). We estimated long-term fatty acid exposure, while reducing measurement error [9], by using fatty acid measures at each time point to generate cumulate averages, weighting the most recent measure at 50% and past measures equally at 25%. For example, The FA value at baseline was related to risk between 1992 and 1998 (the timepoint of the second measure). Then, the average (mean) of the FA value in 1992 and the FA value in 1998 was related to risk between 1998 and 2005 (the timepoint of the third measure). Finally, the average (mean) of the FA values in 1992, 1998, and 2005 (with 50% weight given to the 2005 measure) was related to risk after 2005. This specific approach for cumulative average has been widely used [10–16] to reduce within-subject variation and capture long-term exposure to fatty acids which are more likely to influence cognitive health [9, 17]. For participants with missing PUFA levels, previous measurements were carried forward. Fasting (12 h) blood samples were collected at each study visit, stored at −70°C, and shipped on dry ice for long-term storage at −80°C. The Fred Hutchinson Cancer Research Center Biomarker Laboratory extracted total lipids from plasma using published methods [18]. Separation of phospholipids from neutral lipids was achieved via one-dimensional, thin-layer chromatography. Direct transesterification of phospholipid fractions was used to prepare fatty acid methyl esters, which were subsequently separated by gas chromatography [19]. For 46 fatty acids, quantitative measurements were expressed as percentage of total phospholipid fatty acids. Interassay CVs were less than 5% for each n-3 and n-6 PUFA.
Covariates
CHS clinical visits (1992–1993, 1998–1999, 2005–2006) collected participant demographic characteristics, medical history, prescription medications, anthropometric measures, blood pressure, and laboratory values [8]. A modified Minnesota Leisure-Time Activities questionnaire collected information on the frequency and duration of 15 leisure activities [20]. Participants provided dietary information at baseline via a validated 99-item food-frequency questionnaire adapted from the National Cancer Institute [21], and in 1995–1996 via a validated Willett food-frequency questionnaire [22]. Apolipoprotein E genotype was assessed as previously described [23]. We used single imputation for missing covariate data (<2% for most lifestyle factors; 8–12% for dietary factors), adjusting for demographic and risk factor variables. For data with low missingness, prior analyses in CHS have shown little difference between single and multiple imputation methods [24].
Cognitive Outcomes
Our primary outcomes were cognitive decline, incidence of cognitive impairment, and dementia. Cognitive decline and impairment were assessed based on cognitive testing via the validated Modified Mini-Mental State Examination (3MSE) that assesses memory, orientation, calculation, and verbal fluency [25]. 3MSE tests were administered during annual in-person visits between 1992–1999 and again at the 2005–2006 visit. In addition, cognitive function was assessed using the Telephone Interview for Cognitive Status (TICS) and the Informant Questionnaire on Cognitive Decline in the Elderly informant (IQCODE), administered to a proxy to assess participant decline in cognitive function over the past decade. TICS or IQCODE was collected through 1998/99, at the 2005/06 visit, and from the 2007/2008 visit on (see Supplemental Figure 2). Missing 3MSE scores were imputed using TICS and IQCODE for participants who did not attend study visits (4%–17% each year prior to 2007/8; 100% from the 2007/2008 visit on) following equations developed and validated in CHS [26]. Following cohort recommendations, the analysis of cognitive decline rates included cognitive scores estimated based on 3MSE or TICS measurements (n=2,938), while analysis of incident cognitive impairment included cognitive scores estimated based on 3MSE when available, otherwise using TICS or IQCODE scores (n= 3,291)
Cognitive impairment was defined as having a 3MSE score of 80 or lower on two consecutive visits or on a single visit with no subsequent cognitive information[10, 25]. When assessing associations with incident cognitive impairment, we excluded 274 participants with 3MSE scores <80 at baseline.
Ascertainment and diagnosis of dementia was performed in 3,608 participants free of stroke in 1992–1993 who were included in the 1998–1999 Cardiovascular Health Cognition Study, which was previously described [27, 28]. A participant was identified as having dementia if they had a progressive or static cognitive deficit that affected their activities of daily living, had normal intellectual function before cognitive deficits manifested, or demonstrated impairment in two cognitive domains [27]. Potential dementia cases were initially evaluated at each CHS study site, with subsequent adjudication by a central dementia committee, resulting in 362 dementia cases. Based on the review of MRI data and participant’s neuropsychological tests, the adjudication committee classified incident dementia cases into types [i.e., Alzheimer’s disease (AD), mixed AD, vascular dementia (VD), Parkinson’s disease, other types of dementia], following commonly used guidelines [27, 28]. In addition, we identified dementia cases by using ICD-9 codes from CHS hospitalization data, with at least two ICD codes indicating diagnosis of dementia, Alzheimer’s disease, or related disorders (see Supplemental Table 2). A diagram summarizing the timeline for plasma phospholipids and all cognitive assessments is provided (see Supplemental Figure 2).
Statistical Analysis
We used Tobit models[29] (SAS PROC NLMIXED) to evaluate associations between long-term exposure to circulating PUFA and change in rate of cognitive decline over time. Tobit models were selected based on evidence of ceiling effects for 3MSE scores observed in the present analysis and previously in CHS[30]. Observed when the range of measurement of a certain scale is insufficient to capture differences between individuals scoring at the highest possible values [29], ceiling effects may lead to biased estimates from mixed linear models. The use of Tobit models provide more conservative estimates than those of mixed linear models when ceiling effects are present. We estimated differences in cognitive function associated with interquintile median range units (IQR), (i.e. dividing PUFA measures by the difference between the median FA concentration of the first and fifth quintile of each fatty acid). This allows evaluation of the continuous risk associations in a roughly analogous comparison to the categorical (indicator category) evaluation that compares the top to the bottom quintile. We evaluated associations of each fatty acid separately (using cumulatively-average estimates) and multiplicative interaction terms with each FA and study time in years, with subject-specific intercepts treated as random effects [31].
To evaluate and minimize confounding, we adjusted for all major risk factors related to cognitive outcomes in older adults or associated with PUFA circulating concentrations and/or its dietary sources. Multivariable models included age, sex, race (white or nonwhite), study site, education (years of education through 12th grade, any education beyond 12th grade), income (<$12,000, $12,000-$24,999, $25,000-$49 999, or >$50 000/year), time-varying study time (years), study time-squared (years squared), smoking status (never, current or former smokers), alcohol intake (drinks/week), leisure-time physical activity (kcal/week), intakes of vegetables (servings/day) and fruits (servings/day), plus multiplicative interaction terms for time-varying study time as a linear variable with each covariate.
We applied Cox proportional hazard models to evaluate associations between each n-3 and n-6 PUFA and incident cognitive impairment and dementia. Models included time-at-risk until the first event or censoring including death, clinical stroke (a potentially different endpoint than cognitive decline or dementia), or the last date of adjudicated follow-up [PROC PHREG procedure using counting process for time dependent variables in SAS [32]]. In sensitivity analyses, we repeated the analyses without censoring at time of clinical stroke, given this could be a mediating event in the effect of PUFA on brain health. Fatty acid biomarkers were evaluated in quintiles as indicator variables, and continuously by using IQR units. Trends across quintiles were tested for statistical significance by assigning participants with the median value in each quintile, which was then assessed as a linear variable.
We tested the proportional hazards assumption by using extended Cox models with product terms of each PUFA and model covariates with time-dependent log of time to event or censoring. Evidence of violation of the proportional hazards assumption was observed for the association between circulating AA dementia outcomes (total, and Alzheimer’s Disease), suggesting that the inverse association between cumulative average of circulating AA and dementia grew stronger with time (p-value for interaction term AA variable with log time was 0.018 and 0.03 for total dementia and Alzheimer’s Disease, respectively). Thus, we assessed these associations using extended Cox models including product terms of AA with time-dependent log of time to event. The proportional hazards assumption was rejected for certain covariates including enrollment site and sex, which were corrected by using extended Cox models or by incorporating these as risk-set stratification variables[33]. To evaluate and minimize confounding, we used models sequentially adjusting for pre-specified risk factors associated with cognitive outcomes in older adults. A basic multivariable Cox proportional hazard (model 1) included age (years), age-squared, sex, race (white or nonwhite), and study site; model 2 included additional adjustment for education (years of education through 12th grade, any education beyond 12th grade), income (<$12,000, $12,000-$24,999, $25,000-$49 999, >$50 000/year), APOE ε −4 genotype (at least one ε−4 allele vs none) and most recent information on time-varying smoking status (never, current or former smokers), leisure-time physical activity (kcal/week), and intakes of alcohol (drinks/week), fruits (servings/day), and vegetables (servings/day); and model 3 included additional adjustment for potential mediators of the relationship between circulating PUFA and cognitive function, namely coronary heart disease, atrial fibrillation, and congestive heart failure. We based our interpretation of statistical significance on findings from Model 2, which minimizes confounding by risk factors that are unlikely to be in the causal pathway We explored potential interactions by sex and APOE ε4 genotype by including linear multiplicative interaction terms with each fatty acid level in statistical models.
All P values were two-sided, and P < 0.05 was accepted as significant, except for exploratory subgroup comparisons were Bonferroni-corrected for multiple comparisons (2 effect modifiers x 6 fatty acids = 0.05/12 = 0.0041; see section Potential heterogeneity by APOE ε−4 status and sex). We used Stata, release 12.0 (StataCorp), and SAS version 9.4 (SAS Institute) to perform analyses.
Standard Protocol Approvals, Registrations, and Patient Consents
The Cardiovascular Health Study was approved at the institutional review board of each participating university. All participants provided informed consent.
Results
At baseline, the mean (SD) age was 74.8 (5.1) years, the majority of whom were women (60%), and 12% non-white. Nearly 4% of study participants reported taking fish oil supplements. The mean (SD) body mass index was 26.8 (4.6), with 22% of individuals having coronary heart disease, 3.5 having atrial fibrillation, and 5.4% having heart failure at baseline. LA was the most abundant PUFA (mean ± SD, 19.8 ± 2.5% of total plasma phospholipid fatty acids), followed by AA (11.1 ± 1.3%), DHA (3.0 ± 1.0%), DPA (0.8 ± 0.2%), EPA (0.6 ± 0.4%) and ALA (0.2 ± 0.1%). Intercorrelations across omega-3 PUFA at any time point ranged from 0 to 0.53; were inverse between omega-6 LA and AA (r=−0.53 to −0.64); and were inverse or null between omega-6 and omega-3 PUFA (r=−0.37 to 0) (Supplemental Table 3). Compared to the lowest quintile, participants in the highest quintile of AA were more likely to be non-white and female, to have higher education and income, and to have prevalent CHD; while associations for LA were generally in opposite directions (data not shown).
Cognitive Decline
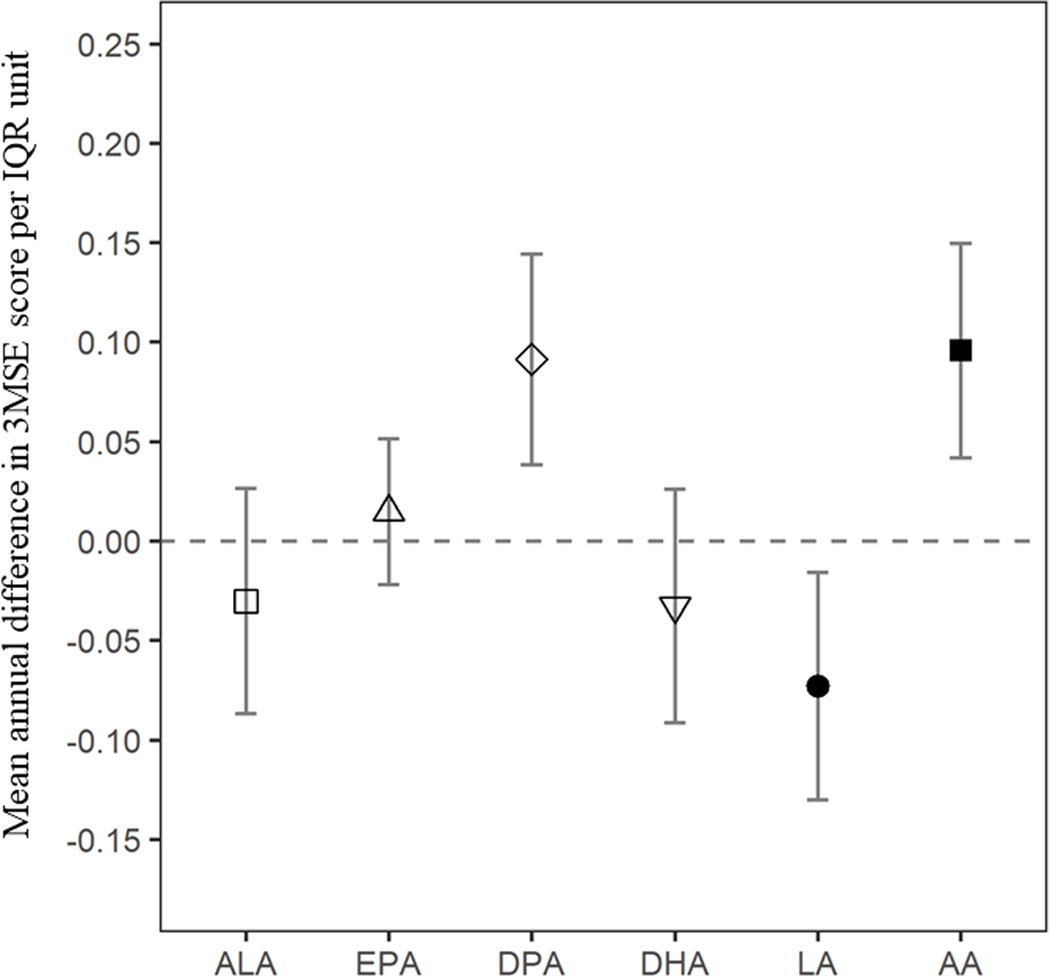
The mean (SD) baseline 100-point 3MSE score was 93.2 (4.8), and the average annual change in cognitive function was approximately −0.5 points per year. When evaluating longitudinal associations of omega-3 PUFA over time, participants with higher plasma phospholipid DPA, had slower decline in cognitive function (unit of higher DPA: +0.09 [0.04, 0.14] per year. Similarly, participants with higher plasma phospholipid AA concentrations had slower cognitive decline (difference [95%CI] in mean cognitive function per IQR of higher AA: +0.10 [0.04, 0.15] per year). Given that the average annual decline in 100-point 3MSE score was approximately 0.5 points per year or 2.5 points over 5 years, these results suggest that over the course of 5 years, people with higher plasma phospholipid DPA (1-IQR unit or 0.42% higher circulating DPA) or AA (1-IQR unit: or 4.9% higher circulating AA) would experience on average nearly 0.5 point less decline, or the equivalent of 1 less year of cognitive aging over the 5 years. On the other hand, mean 3MSE scores declined faster in participants with higher concentrations of plasma phospholipid LA (per IQR of higher LA: −0.07 [−0.013, −0.02]). There were no statistically significant associations between ALA, EPA or DHA and changes in cognitive function over time (Table 2, Figure 1).
Table 2.
Associations between circulating omega-3 and omega-6 fatty acids and cognitive decline among 2,938 older US adults free of cognitive impairment and stroke at baseline
| β (95%CI) | p-value | |
|---|---|---|
| Omega-3 Fatty Acids | ||
| ALA* | 0.36 (−0.18, 0.89) | 0.19 |
| Time × ALA** | −0.03 (−0.09, 0.03) | 0.30 |
| EPA* | 0.67 (0.26, 1.08) | 0.001 |
| Time × EPA** | 0.01 (−0.02, 0.05) | 0.43 |
| DPA* | 0.47 (−0.11, 1.04) | 0.11 |
| Time × DPA** | 0.09 (0.04, 0.14) | <0.001 |
| DHA* | 1.46 (0.83, 2.10) | <.0001 |
| Time × DHA** | −0.03 (−0.09, 0.03) | 0.28 |
| Omega-6 Fatty Acids | ||
| LA* | 0.22 (−0.39, 0.84) | 0.47 |
| Time × LA** | −0.07 (−0.13, −0.02) | 0.013 |
| AA* | −0.18 (−0.81, 0.45) | 0.57 |
| Time × AA** | 0.10 (0.04, 0.15) | <0.001 |
Tobit models for repeated circulating fatty acid measures assessed continuously using IQR units, with intercept as random, adjusted for baseline age, sex, race (white or nonwhite), study site, education (years of education through 12th grade, any education beyond 12th grade), income (<$12,000, $12,000-$24,999, $25,000-$49 999, or >$40 000/year), time-varying study time (years), study time squared (years2), smoking status (never, current or former smokers), alcohol intake (drinks/week), leisure-time physical activity (kcal/week), intakes of vegetables (servings/day) and fruits (servings/day), plus multiplicative interaction terms for follow-up time as continuous variable with each covariate. 1-IQR=0.124 (circulating ALA), 0.63 (circulating EPA), 0.42 (circulating DPA), 2.4 (circulating DHA), 2.9 (circulating EPA+DPA+DHA), 6.3 (circulating LA), 4.9 (circulating AA).
Interpreted as 3MSE difference at baseline associated with 1 IQR-unit of plasma phospholipid fatty acid concentration.
Interpreted as annual change in cognitive function associated with 1-IQR unit of plasma phospholipid fatty acid concentration.
Abbreviations: AA, arachidonic acid; ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; IQR, interquintile median range.
Figure 1. Mean difference in annual rate of cognitive decline 3MSE scores for 1-IQR unit difference in plasma phospholipid fatty acids.
Legend: Positive values indicate slower decline, while negative values suggest faster decline in cognitive function. Mean values were estimated using Tobit Models adjusted for baseline age, sex, race (white or nonwhite), study site, education (years of education through 12th grade, any education beyond 12th grade), income (<$12,000, $12,000-$24,999, $25,000-$49 999, or >$50 000/year), time-varying study time (years), study time2 (years2), smoking status (never, current or former smokers), alcohol intake (drinks/week), leisure-time physical activity (kcal/week), intakes of vegetables (servings/day) and fruits (servings/day), plus multiplicative interaction terms for follow-up time as continuous variable with each covariate.
Abbreviations: AA, arachidonic acid; ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid.
Incident Cognitive Impairment (Binary) and Dementia
During a maximum of 23 years of follow-up (mean follow-up time: 11.2 years), 507 new cases of cognitive impairment (13.4 cases per 1,000 person-years) and 499 new dementia cases (12.5 per 1,000 person-years) were identified. In multivariable models adjusting for sociodemographic, lifestyle, cardiovascular, and dietary factors, plasma phospholipid DPA, but not other omega-3 or omega-6 fatty acids, was associated with lower risk of incident cognitive impairment evaluated as a binary variable (HR [95% CI] for each IQR difference increase of circulating DPA was 0.79 [0.63 to 0.99]); Table 3).
Table 3.
Risk of incident cognitive outcomes according to quintiles of serial measures of circulating omega-3 and omega-6 Fatty Acids1 in free stroke and dementia at baseline
| Quintiles of Plasma Phospholipid Fatty Acid | P-trend | Hazard Ratio (95% CI) for 1-IQR unit2 | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Cognitive Impairment3 (n=3,291; 507 events) | |||||||
|
| |||||||
| Omega-3 Fatty Acids | |||||||
| ALA | |||||||
| Model 13 | Reference | 0.87 (0.65, 1.16) | 0.75 (0.56, 1.01) | 0.75 (0.57, 0.99) | 0.76 (0.57, 1.02) | 0.09 | 0.82 (0.65, 1.04) |
| Model 2 | Reference | 0.89 (0.66, 1.20) | 0.79 (0.59, 1.06) | 0.84 (0.63, 1.11) | 0.86 (0.65, 1.15) | 0.42 | 0.93 (0.73, 1.18) |
| Model 3 | Reference | 0.89 (0.66, 1.19) | 0.80 (0.60, 1.08) | 0.85 (0.64, 1.13) | 0.87 (0.65, 1.17) | 0.47 | 0.94 (0.74, 1.19) |
| EPA | |||||||
| Model 1 | Reference | 1.03 (0.76, 1.38) | 0.82 (0.61, 1.10) | 0.86 (0.65, 1.15) | 0.69 (0.51, 0.93) | 0.01 | 0.84 (0.70, 1.01) |
| Model 2 | Reference | 1.06 (0.79, 1.43) | 0.89 (0.66, 1.19) | 0.99 (0.74, 1.33) | 0.81 (0.60, 1.11) | 0.14 | 0.93 (0.77, 1.11) |
| Model 3 | Reference | 1.11 (0.82, 1.49) | 0.92 (0.68, 1.23) | 1.03 (0.76, 1.38) | 0.84 (0.62, 1.15) | 0.18 | 0.94 (0.78, 1.12) |
| DPA | |||||||
| Model 1 | Reference | 0.69 (0.52, 0.92) | 0.64 (0.49, 0.84) | 0.59 (0.45, 0.79) | 0.71 (0.54, 0.93) | 0.02 | 0.79 (0.63, 0.99) |
| Model 2 | Reference | 0.73 (0.55, 0.96) | 0.66 (0.50, 0.86) | 0.62 (0.47, 0.82) | 0.72 (0.55, 0.95) | 0.03 | 0.79 (0.63, 0.99) |
| Model 3 | Reference | 0.73 (0.55, 0.97) | 0.67 (0.51, 0.88) | 0.64 (0.48, 0.85) | 0.75 (0.57, 0.98) | 0.06 | 0.82 (0.66, 1.04) |
| DHA | |||||||
| Model 1 | Reference | 0.84 (0.63, 1.11) | 0.76 (0.58, 1.01) | 0.69 (0.52, 0.92) | 0.72 (0.54, 0.94) | 0.02 | 0.79 (0.63, 1.00) |
| Model 2 | Reference | 0.81 (0.61, 1.07) | 0.80 (0.60, 1.06) | 0.76 (0.57, 1.03) | 0.80 (0.60, 1.07) | 0.21 | 0.90 (0.71, 1.16) |
| Model 3 | Reference | 0.83 (0.62, 1.10) | 0.79 (0.60, 1.05) | 0.77 (0.57, 1.03) | 0.81 (0.60, 1.09) | 0.22 | 0.90 (0.70, 1.16) |
| EPA+DPA+DHA | |||||||
| Model 1 | Reference | 0.86 (0.65, 1.14) | 0.78 (0.59, 1.05) | 0.68 (0.51, 0.91) | 0.71 (0.54, 0.94) | 0.02 | 0.77 (0.62, 0.96) |
| Model 2 | Reference | 0.89 (0.67, 1.18) | 0.80 (0.60, 1.08) | 0.78 (0.58, 1.04) | 0.83 (0.62, 1.12) | 0.28 | 0.87 (0.69, 1.10) |
| Model 3 | Reference | 0.90 (0.68, 1.20) | 0.80 (0.60, 1.07) | 0.80 (0.59, 1.07) | 0.84 (0.63, 1.14) | 0.32 | 0.88 (0.70, 1.11) |
| Omega-6 Fatty Acids | |||||||
| LA | |||||||
| Model 1 | Reference | 1.07 (0.81, 1.40) | 1.02 (0.77, 1.34) | 1.13 (0.85, 1.50) | 1.13 (0.85, 1.50) | 0.37 | 1.07 (0.84, 1.36) |
| Model 2 | Reference | 1.04 (0.79, 1.37) | 1.03 (0.78, 1.36) | 1.11 (0.83, 1.48) | 1.17 (0.87, 1.56) | 0.28 | 1.10 (0.86, 1.41) |
| Model 3 | Reference | 1.03 (0.78, 1.36) | 1.03 (0.77, 1.36) | 1.08 (0.81, 1.45) | 1.14 (0.86, 1.53) | 0.35 | 1.09 (0.84, 1.39) |
| AA | |||||||
| Model 1 | Reference | 0.86 (0.65, 1.15) | 0.90 (0.68, 1.19) | 0.85 (0.65, 1.13) | 0.85 (0.65, 1.13) | 0.30 | 0.85 (0.68, 1.08) |
| Model 2 | Reference | 0.85 (0.64, 1.13) | 0.90 (0.68, 1.19) | 0.84 (0.63, 1.11) | 0.82 (0.62, 1.09) | 0.20 | 0.81 (0.64, 1.02) |
| Model 3 | Reference | 0.86 (0.65, 1.15) | 0.93 (0.70, 1.23) | 0.86 (0.65, 1.14) | 0.84 (0.63, 1.11) | 0.26 | 0.82 (0.65, 1.04) |
|
| |||||||
| Total Dementia3,4 (n=3,564; 499 events) | |||||||
|
| |||||||
| Omega-3 Fatty Acids | |||||||
| ALA | |||||||
| Model 13 | Reference | 0.90 (0.68, 1.20) | 0.88 (0.67, 1.17) | 0.78 (0.58, 1.03) | 0.93 (0.71, 1.23) | 0.51 | 1.02 (0.82, 1.26) |
| Model 2 | Reference | 0.90 (0.67, 1.19) | 0.89 (0.67, 1.17) | 0.80 (0.60, 1.07) | 0.95 (0.72, 1.26) | 0.67 | 1.04 (0.84, 1.29) |
| Model 3 | Reference | 0.89 (0.67, 1.18) | 0.89 (0.67, 1.18) | 0.80 (0.60, 1.06) | 0.95 (0.72, 1.26) | 0.67 | 1.05 (0.84, 1.30) |
| EPA | |||||||
| Model 1 | Reference | 1.03 (0.78, 1.37) | 0.97 (0.74, 1.29) | 0.69 (0.51, 0.93) | 0.98 (0.74, 1.31) | 0.50 | 1.00 (0.85, 1.17) |
| Model 2 | Reference | 1.03 (0.78, 1.37) | 0.98 (0.74, 1.30) | 0.70 (0.52, 0.95) | 1.03 (0.77, 1.38) | 0.78 | 1.03 (0.88, 1.21) |
| Model 3 | Reference | 1.05 (0.79, 1.39) | 1.00 (0.76, 1.32) | 0.72 (0.53, 0.98) | 1.05 (0.78, 1.40) | 0.86 | 1.04 (0.89, 1.21) |
| DPA | |||||||
| Model 1 | Reference | 0.92 (0.68, 1.24) | 0.86 (0.64, 1.16) | 1.00 (0.75, 1.33) | 1.04 (0.79, 1.38) | 0.51 | 1.03 (0.82, 1.29) |
| Model 2 | Reference | 0.92 (0.68, 1.24) | 0.88 (0.65, 1.18) | 1.03 (0.78, 1.37) | 1.05 (0.79, 1.40) | 0.43 | 1.03 (0.83, 1.29) |
| Model 3 | Reference | 0.91 (0.68, 1.23) | 0.89 (0.66, 1.19) | 1.04 (0.79, 1.39) | 1.08 (0.81, 1.43) | 0.33 | 1.06 (0.85, 1.32) |
| DHA | |||||||
| Model 1 | Reference | 1.05 (0.79, 1.39) | 1.10 (0.83, 1.45) | 0.85 (0.63, 1.15) | 0.96 (0.72, 1.28) | 0.47 | 1.03 (0.82, 1.29) |
| Model 2 | Reference | 1.07 (0.80, 1.43) | 1.11 (0.84, 1.46) | 0.88 (0.65, 1.19) | 1.01 (0.75, 1.36) | 0.74 | 1.09 (0.86, 1.38) |
| Model 3 | Reference | 1.11 (0.83, 1.47) | 1.13 (0.86, 1.49) | 0.90 (0.67, 1.21) | 1.02 (0.76, 1.38) | 0.75 | 1.08 (0.85, 1.37) |
| EPA+DPA+DHA | |||||||
| Model 1 | Reference | 0.90 (0.68, 1.19) | 0.85 (0.64, 1.13) | 0.75 (0.56, 1.00) | 0.88 (0.67, 1.17) | 0.36 | 1.02 (0.83, 1.26) |
| Model 2 | Reference | 0.92 (0.69, 1.21) | 0.86 (0.64, 1.14) | 0.78 (0.58, 1.04) | 0.94 (0.70, 1.25) | 0.63 | 1.08 (0.87, 1.34) |
| Model 3 | Reference | 0.95 (0.72, 1.26) | 0.87 (0.65, 1.15) | 0.80 (0.59, 1.07) | 0.95 (0.71, 1.27) | 0.66 | 1.08 (0.87, 1.34) |
| Omega-6 Fatty Acids | |||||||
| LA | |||||||
| Model 1 | Reference | 1.10 (0.83, 1.45) | 0.92 (0.68, 1.23) | 1.11 (0.84, 1.48) | 1.02 (0.76, 1.37) | 0.91 | 0.96 (0.76, 1.23) |
| Model 2 | Reference | 1.10 (0.83, 1.45) | 0.93 (0.70, 1.25) | 1.13 (0.85, 1.51) | 1.05 (0.78, 1.42) | 0.71 | 1.00 (0.78, 1.27) |
| Model 3 | Reference | 1.10 (0.83, 1.46) | 0.94 (0.70, 1.26) | 1.13 (0.85, 1.51) | 1.05 (0.78, 1.41) | 0.76 | 0.99 (0.78, 1.27) |
|
| |||||||
| Alzheimer’s Disease only3 (n=3,564; 465 events) | |||||||
|
| |||||||
| Omega-3 Fatty Acids | |||||||
| ALA | |||||||
| Model 13 | Reference | 0.86 (0.64, 1.15) | 0.82 (0.61, 1.10) | 0.75 (0.56, 1.00) | 0.81 (0.61, 1.09) | 0.15 | 0.95 (0.76, 1.19) |
| Model 2 | Reference | 0.85 (0.63, 1.14) | 0.83 (0.62, 1.12) | 0.77 (0.58, 1.04) | 0.84 (0.62, 1.12) | 0.25 | 0.98 (0.78, 1.23) |
| Model 3 | Reference | 0.84 (0.63, 1.13) | 0.84 (0.63, 1.13) | 0.78 (0.58, 1.04) | 0.84 (0.63, 1.13) | 0.27 | 0.99 (0.79, 1.24) |
| EPA | |||||||
| Model 1 | Reference | 0.87 (0.65, 1.17) | 0.85 (0.64, 1.14) | 0.62 (0.46, 0.85) | 0.85 (0.63, 1.13) | 0.21 | 0.95 (0.79, 1.13) |
| Model 2 | Reference | 0.85 (0.63, 1.14) | 0.85 (0.64, 1.14) | 0.63 (0.46, 0.86) | 0.87 (0.64, 1.18) | 0.35 | 0.97 (0.81, 1.16) |
| Model 3 | Reference | 0.86 (0.64, 1.16) | 0.87 (0.65, 1.16) | 0.65 (0.47, 0.88) | 0.89 (0.66, 1.20) | 0.41 | 0.98 (0.82, 1.17) |
| DPA | |||||||
| Model 1 | Reference | 0.82 (0.60, 1.12) | 0.81 (0.60, 1.10) | 0.96 (0.72, 1.28) | 0.98 (0.73, 1.31) | 0.64 | 0.96 (0.76, 1.22) |
| Model 2 | Reference | 0.82 (0.60, 1.12) | 0.83 (0.61, 1.12) | 0.97 (0.72, 1.29) | 0.98 (0.74, 1.32) | 0.61 | 0.96 (0.76, 1.21) |
| Model 3 | Reference | 0.82 (0.60, 1.12) | 0.84 (0.62, 1.14) | 0.98 (0.73, 1.31) | 1.01 (0.75, 1.35) | 0.47 | 0.99 (0.78, 1.25) |
| DHA | |||||||
| Model 1 | Reference | 1.05 (0.78, 1.41) | 1.17 (0.88, 1.55) | 0.83 (0.61, 1.13) | 0.93 (0.69, 1.25) | 0.31 | 0.97 (0.77, 1.23) |
| Model 2 | Reference | 1.07 (0.80, 1.44) | 1.17 (0.88, 1.56) | 0.84 (0.61, 1.15) | 0.96 (0.70, 1.31) | 0.45 | 1.01 (0.79, 1.30) |
| Model 3 | Reference | 1.11 (0.82, 1.49) | 1.20 (0.90, 1.59) | 0.85 (0.62, 1.16) | 0.97 (0.71, 1.32) | 0.45 | 1.01 (0.79, 1.29) |
| EPA+DPA+DHA | |||||||
| Model 1 | Reference | 0.92 (0.69, 1.24) | 0.99 (0.74, 1.32) | 0.79 (0.58, 1.06) | 0.89 (0.66, 1.19) | 0.33 | 0.96 (0.77, 1.19) |
| Model 2 | Reference | 0.94 (0.70, 1.26) | 0.99 (0.73, 1.32) | 0.81 (0.59, 1.10) | 0.93 (0.68, 1.26) | 0.52 | 0.99 (0.79, 1.25) |
| Model 3 | Reference | 0.98 (0.73, 1.32) | 1.00 (0.75, 1.35) | 0.83 (0.61, 1.13) | 0.94 (0.69, 1.28) | 0.56 | 1.00 (0.79, 1.26) |
| Omega-6 Fatty Acids | |||||||
| LA | |||||||
| Model 1 | Reference | 1.07 (0.80, 1.43) | 1.02 (0.76, 1.37) | 1.10 (0.82, 1.48) | 1.06 (0.78, 1.44) | 0.68 | 0.98 (0.76, 1.26) |
| Model 2 | Reference | 1.06 (0.79, 1.43) | 1.04 (0.77, 1.40) | 1.13 (0.84, 1.52) | 1.11 (0.81, 1.50) | 0.48 | 1.03 (0.80, 1.32) |
| Model 3 | Reference | 1.06 (0.79, 1.43) | 1.03 (0.77, 1.39) | 1.12 (0.83, 1.51) | 1.09 (0.80, 1.48) | 0.54 | 1.01 (0.79, 1.30) |
long-term exposure was assessed by using cumulative average of fatty acid measures, i.e., FA levels in 1992 were related to risk from 1992–98; the average of FA levels in 1992 and 1998, to risk from 1998–2005; and the weighted average of FA levels in 1992, 1998, and 2005, to risk after 2005, with 50% weight assigned to the most recent measurement, and 25% assigned to each previous measurement. Associations of AA with total dementia and Alzheimer’s disease are shown in Table 3.
1-IQR=0.124 (circulating ALA), 0.63 (circulating EPA), 0.42 (circulating DPA), 2.4 (circulating DHA), 2.9 (circulating EPA+DPA+DHA), 6.3 (circulating LA), 4.9 (circulating AA).
Multivariate model 1 included sex, race (whites or nonwhite) and enrollment site (4 sites) and time-varying age (years), and age-squared ; Model 2 included additional adjustment for education (years of education through 12th grade, any education beyond 12th grade), income (<$12 000, $12 000-$24 999, $25 000-$49 999, or >$50 000/year), APOE status (at least one ε−4 vs none), and time-varying information on smoking status (never, current or former smokers), alcohol intake (drinks/week), leisure-time physical activity (kcal/week), vegetable and fruit intake (servings/day), Model 3 included additional adjustment for time-varying prevalent CHD, atrial fibrillation and heart failure. Time-varying covariates were updated at each fatty acid measurement
Abbreviations: AA, arachidonic acid; ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid, IQR, interquintile median range.
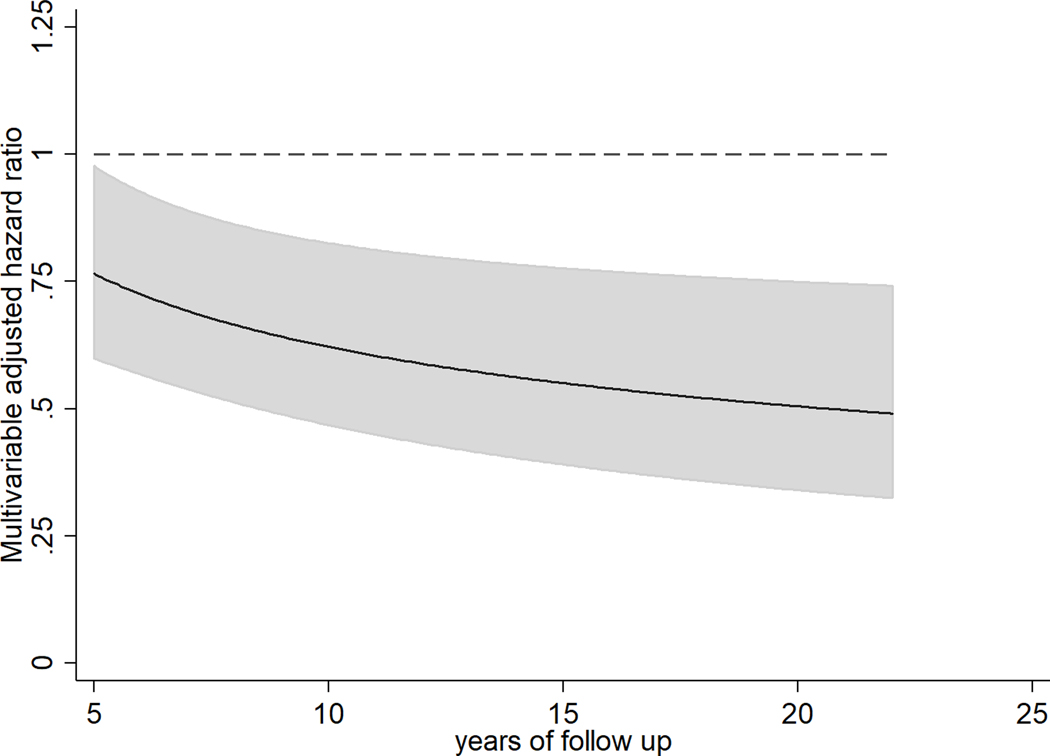
When evaluating clinical dementia, higher plasma phospholipid AA concentrations were associated with lower risk, with the inverse association growing stronger with increasing follow-up time. For example, the HR (95% CI) per IQR of AA was 0.77 (0.60, 0.98) at 5 years, 0.62 (0.47,0.83) at 10 years, and 0.55 (0.39,0.78) at 15 years of follow-up (P-interaction with time = 0.018) (Figure 2). Relative risks for the association between circulating AA and dementia risk were mostly unchanged when adjusting for other n-3 and n-6 PUFA (i.e. ALA, EPA+DPH+DHA and LA), the HR (95% CI per IQR of AA was 0.74 (0.56,0.97) at 5 years, 0.60 (0.44,0.82) at 10 years, and 0.53 (0.37,0.77) at 15 years of follow-up. No statistically significant associations were seen between plasma phospholipid omega-3 PUFA or LA with dementia risk. Associations with cognitive outcomes were similar after adjustment for fish oil supplements (data not shown), used by 4% of participants at baseline. Further adjustment for potential mediators including time-varying prevalent coronary heart disease, atrial fibrillation, and congestive heart failure did not materially alter results (Table 3). In addition, no differences were observed after adjustment for medication use (lipid lowering and hypertension medication), cardiovascular risk factors (i.e. circulating total cholesterol, triglycerides), and subclinical atherosclerosis (i.e. intima-media thickness of the common carotid artery) (data not shown). Among dementia subtypes, AA was inversely associated with Alzheimer’s disease. Similar to associations with total dementia, this inverse association grew stronger overtime. For example, the HR (95% CI) per IQR of AA was 0.83 (0.64, 1.08) at 5 years, 0.67 (0.51,0.88) at 10 years, and 0.59 (0.42,0.81) at 15 years of follow-up (P-interaction with time = 0.03) (Table 4).
Figure 2. Multivariable-adjusted hazard ratios (and 95% confidence intervals) of total dementia (n=3,564) for 1-IQR unit of circulating AA in older US adults free of dementia and stroke at baseline. Solid lines and shaded area represent the HR estimate and 95% confidence interval overtime, respectively.
Legend: Long-term exposure was assessed by using cumulative average of serial fatty acid measures, i.e., FA levels in 1992 were related to risk from 1992–98; the average of FA levels in 1992 and 1998, to risk from 1998–2005; and the weighted average of FA levels in 1992, 1998, and 2005, to risk after 2005, with 50% weight assigned to the most recent measurement, and 25% assigned to each previous measurement.
The solid line and grey area represent hazard ratios and 95%CI estimated using and , where is the estimated coefficient for 1-IQR unit of AA, and is the estimated coefficient for the multiplicative interaction term (i.e. AA x log(time)), t is years of follow-up, and are estimated variances and covariance for and . Time-varying covariates were updated at each fatty acid measurement.
Multivariable model included adjustment for sex, race (whites or nonwhite), enrollment site (4 sites), education (years of education through 12th grade, any education beyond 12th grade), income (<$12 000, $12 000-$24 999, $25 000-$49 999, or >$50 000/year), APOE status (at least one ε−4, none, unknown), time-varying information on age (years), age-squared, smoking status (never, current or former smokers), alcohol intake (drinks/week), leisure-time physical activity (kcal/week), vegetable (servings/day), fruit intake (servings/day), and a multiplicative interaction term AA as linear variable with log of time.
Table 4:
Risk of total dementia and Alzheimer’s Disease for 1-IQR unit of AA1 in 3,564 older US adults free of stroke at baseline
| Hazard Ratio (95% CI) for 1-IQR unit | ||
|---|---|---|
|
| ||
| Years of Follow-up | Dementia (499 new cases) | Alzheimer’s Disease (465 new cases) |
| 5.0 | 0.77 (0.60, 0.98) | 0.83 (0.64, 1.08) |
| 7.5 | 0.68 (0.53, 0.88) | 0.73 (0.57, 0.94) |
| 10.0 | 0.62 (0.47, 0.83) | 0.67 (0.51, 0.88) |
| 12.5 | 0.58 (0.42, 0.80) | 0.62 (0.46, 0.84) |
| 15.0 | 0.55 (0.39, 0.78) | 0.59 (0.42, 0.81) |
| 17.5 | 0.53 (0.36, 0.76) | 0.56 (0.39, 0.79) |
| 20.0 | 0.51 (0.34, 0.75) | 0.54 (0.37, 0.78) |
long-term exposure was assessed by using cumulative average of fatty acid measures, i.e., FA levels in 1992 were related to risk from 1992–98; the average of FA levels in 1992 and 1998, to risk from 1998–2005; and the weighted average of FA levels in 1992, 1998, and 2005, to risk after 2005, with 50% weight assigned to the most recent measurement, and 25% assigned to each previous measurement.
1-interquartile median range (IQR) of circulating AA=4.9%.
Multivariate model included multiplicative interaction term AA as continuous variable with log of time to dementia, sex, race (whites or nonwhite) and enrollment site (4 sites) and time-varying age (years), and age-squared, education (years of education through 12th grade, any education beyond 12th grade), income (<$12 000, $12 000-$24 999, $25 000-$49 999, or >$50 000/year), APOE status (at least one ε−4 vs none), and time-varying information on smoking status (never, current or former smokers), alcohol intake (drinks/week), leisure-time physical activity (kcal/week), vegetable and fruit intake (servings/day).
Abbreviations: IQR interquintile median range; AA, arachidonic acid
Potential heterogeneity by APOE ε−4 status and sex
In exploratory analyses in the subset of participants with available APOE ε−4 genotype (n=3,284), all associations of circulating omega-3 and omega-6 PUFAs were similar by APOE ε−4 status (Bonferroni-corrected P-value >0.0041), except for the association between plasma phospholipid AA and incident dementia, where multivariable-adjusted HR (95% CI) per IQR was 1.04 (0.64 to 1.69) and 0.50 (0.43 to 0.59) in individuals with at least one APOE e4 allele vs none at 10 years of follow up, respectively (p-value for interaction = 0.0005). Significant differences in associations of circulating AA by APOE status were also observed when evaluating associations with Alzheimer’s Disease (95% CI) per IQR was 1.12 (0.72 to 1.74) and 0.45 (0.45 to 0.66) in individuals with at least one APOE e4 allele vs none at 10 years of follow up, respectively (p-value for interaction = 0.001). Directions and magnitudes of all associations were generally similar in men and women.
Sensitivity Analysis
Findings were generally similar in analyses not censoring on clinical stroke (data not shown), with the exception that the inverse association between circulating AA and dementia was slightly weaker, with a HR (95% CI) per 1-IQR unit of 0.83 (0.67,1.02) at 5 years, 0.69 (0.53,0.89) at 10 years, and 0.62 (0.45,0.84) at 15 years of follow-up. In addition, associations between AA and cognitive function were generally similar when we used the most recent measured AA concentrations (difference [95%CI] in mean cognitive function per IQR of higher AA: +0.06 [0.01, 0.11] per year).
Discussion
In this large prospective investigation of older US adults, we evaluated the associations of serial measures of plasma phospholipid omega-3 and omega-6 PUFA biomarkers with rates of cognitive decline and risk of incident cognitive impairment and dementia. Circulating concentrations of AA and DPA were associated with a slower rate of cognitive decline. Higher DPA concentrations were associated with a lower risk of cognitive impairment, while AA was associated with lower risk of incident dementia. Associations between AA and dementia were stronger with greater length of follow-up and stronger for Alzheimer’s disease.
Findings were generally null or inconsistent for other PUFAs. LA was associated with a faster decline in cognitive function, but was not associated with incident cognitive impairment evaluated as a binary variable or with risk of clinical dementia. ALA, EPA and DHA were not significantly associated with any of these outcomes. These findings provide a comprehensive assessment, for the first time to our knowledge, using serial measures of circulating fatty acids in a large, prospective cohort of older adults with well-phenotyped cognitive outcomes.
AA, a key omega-6 PUFA, is a major structural component of membrane lipids of the nervous system, representing nearly 20% of all fatty acids in the brain [1, 34]. However, its potential effects on cognitive function remain mostly unknown. Although AA is present in animal foods and fish, circulating levels of these fatty acids are not strongly correlated with diet [35–37], suggesting that circulating AA levels are tightly regulated endogenously. Surprisingly, despite its being the most predominant PUFA in the brain, we identified very few experimental or intervention studies evaluating AA and its effects on cognition [38–41], and only one included older adults – the Supplementation with Antioxidant Vitamins and Minerals study (SAVM). In that study among 3362 older adults (mean age at baseline, 65.5 years), an inverse association was seen between self-reported estimates of dietary AA consumption and cognitive function among participants in the placebo group, and no association was seen among participants taking antioxidant supplements [38].
AA can be metabolized by cyclooxygenase (COX) and lipoxygenase (LOX), generating leukotrienes and prostaglandins which have traditionally been thought to be pro-inflammatory [1]. On the other hand, AA is also metabolized by lipoxygenase into specialized pro-resolving mediators (SPMs), particularly lipoxin LXA4[42], which appear to play a critical role in the resolution of acute inflammation [1, 43]. The observed slower decline in cognitive function and the inverse association with onset of dementia support potential long-term benefits of higher AA levels for cognitive outcomes later in life. Our findings cannot determine whether potential protective associations could be attributed to AA itself, to its downstream bioactive metabolites, or to metabolic processes influencing plasma phospholipid AA levels. Our novel findings emphasize the need to further elucidate specific pathways related to circulating AA that could influence and be effective in preserving cognitive function later in life.
Overall, our findings do not provide evidence for major associations of omega-3 fatty acids DHA, EPA or ALA with cognitive outcomes among older adults. These results are consistent with a meta-analysis of 7 randomized clinical trials including 585 healthy individuals showing no significant effects of omega-3 fatty acid supplementation on cognitive function, assessed using MMSE scores, or other cognitive domains such as attention, processing speed, delayed recall or memory among healthy individuals [44]. Post-hoc analyses of two randomized trials suggest potential complementary interactions between omega-3 status and B vitamin status[45, 46]; such a possibility requires more study. Prior observational studies also do not provide strong support for beneficial associations of circulating omega-3 PUFA with cognitive impairment or dementia [47–51], although two studies found inverse associations between seafood-derived EPA or DHA, not DPA, and risk of dementia [47, 48]. All these previous studies included predominantly middle-aged adults and were limited to a single baseline fatty acid measure. The observed inverse associations of DPA support the need to investigate mechanisms underlying potential effects of DPA on brain health, particularly later in life. Our findings expand upon and greatly extend these previous results by evaluating serial circulating fatty acid measures over 13 years and including older adults, the age at which rates of cognitive decline and dementia are greatest; and by evaluating a continuum of cognitive outcomes from early decline in cognitive function to incident dementia.
Our study has several strengths. The use of serial biomarker measurements provided objective measures of long-term exposure to PUFA, minimizing recall bias and measurement error due to changes in exposure over time; and allowing assessment of different specific PUFA. The prospective cohort design established temporality and reduced the possibility of reverse causation. Multivariable adjustment for major risk factors at the time of each fatty acid measurement minimized the influence of confounding. Censoring participants at the time of stroke allowed us to evaluate associations with cognitive outcomes in the absence of clinical stroke.
Our study also had potential limitations. The use of three serial fatty acid measures may not have fully captured all changes in fatty acid concentrations over 23y of follow up. Also, we acknowledge that the use of last observation carried forward method to impute missing fatty acid values may reduce the apparent variability of the data. We cannot exclude the possibility of residual confounding by unmeasured or imprecisely measured confounders. Given the known correlation, measurement of circulating fatty acids provide an estimate of their levels in the brain [52]. However, assessment of circulating PUFA biomarkers cannot account for other potentially relevant processes such as their conversion to bioactive metabolites in the brain[53]. Missing 3MSE scores and our handling of such data could result in underestimation of cognitive decline in our study population. In addition, some dementia outcomes may have been misclassified, which would probably be random with respect to fatty acid measures and may lead to potential attenuation of true associations toward the null.Finally, as it has been pointed out by Rothman, there are challenges and limitations to adjustment of test statistics to account for multiple comparisons when evaluating distinct hypothesis in the same analysis[54]. In order to minimize concerns related to type I error, we 1) tested associations with prespecified hypotheses focusing on statistical significance of a single defined model (model 2) evaluating each relationship, and 2) interpreted our results in the light of consistency with previous observations, biological plausibility and construct validity of the method used.
In summary, our prospective investigation of older adults suggests a protective association of circulating AA and DPA with the rate of cognitive decline and onset of total dementia, Alzheimer’s disease or cognitive impairment, without consistent associations of circulating LA or omega-3 PUFA with cognitive function later in life.
Supplementary Material
Table 1.
Baseline characteristics of 3,564 US adults free of dementia and stroke at baseline in the Cardiovascular Health Study1
| Sociodemographic Factors | |
| Mean age (SD), years | 74.8 (5.1) |
| White, n (%) | 3,146 (88) |
| Male, n (%) | 1,413 (40) |
| High School or higher, n (%) | 2,661 (75) |
| Annual income, n (%) | |
| <12,000 | 792 (22) |
| $12,000-$24,999 | 1,281 (36) |
| $25,000-$49,999 | 995 (28) |
| >$50,000 | 496 (14) |
| Health Status, n (%) | |
| Mean Body Mass Index (SD), kg/m2 | 26.8 (4.6) |
| Coronary Heart Disease, n (%) | 767 (22) |
| Atrial Fibrillation, n (%) | 125 (3.5) |
| Heart Failure, n (%) | 193 (5.4) |
| Lifestyle | |
| Former Smoker, n (%) | 1,507 (42) |
| Current Smoker, n (%) | 369 (10) |
| Alcohol, drinks/week | 2.6 (6.4) |
| Mean Physical activity (SD), kcal/week | 1,180 (1,371) |
| Dietary Risk Factors | |
| Mean intake of fruits (SD), serving/day | 2.2 (1.0) |
| Mean intake of vegetables (SD), serving/day | 3.0 (1.4) |
| Mean intake of fish (SD), serving/week | 1.7 (1.3) |
| Dietary Supplement Use, n (%) | 918 (26) |
| Fish oil Supplement Use, n (%) | 136 (3.8) |
| Mean (SD) Circulating PUFAs, % of total phospholipid fatty acids | |
| ALA | 0.15 (0.05) |
| EPA | 0.6 (0.4) |
| DPA | 0.8 (0.2) |
| DHA | 3.0 (1.0) |
| LA | 19.8 (2.5) |
| AA | 11.1 (1.3) |
| EPA+DPA+DHA | 4.5 (1.3) |
Values are mean (SD) for continuous variables and percent for categorical variables. AA indicates arachidonic acid; ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; MET, metabolic equivalent; PUFA, polyunsaturated fatty acid; SD, standard deviation.
Supplements of calcium or potassium
Acknowledgements.
We thank Nicolas Gargurevich and Hyunkyoung Kim for their assistance preparing tables and figures. We also thank the other investigators, staff, and all CHS participants for their important contributions. A full list of participating CHS investigators and institutions can be found at www.chs-nhlbi.org.
Funding.
Supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging. MCdOO was supported by NIH/NHLBI R01HL085710 and NIH/NHLBI 3R01HL085710–07S1;DM was supported by NIH/NHLBI R01HL085710, NIH/NHLBI R01HL115189, NIH/NHLBI R01HL130735, and NIH/NHLBI R01HL135920; the Bill & Melinda Gates Foundation (OPP1099505); and the Federal Emergency Management Agency (EMW-2014-FP-00612).
Study Funding:
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). MCOO was supported by NIH/NHLBI R01HL085710 and NIH/NHLBI 3R01HL085710–07S1; DM was supported by NIH/NHLBI R01HL085710, NIH/NHLBI R01HL115189, NIH/NHLBI R01HL130735, NIH/NHLBI R01HL135920, Bill & Melinda Gates Foundation OPP1099505, FEMA EMW-2014-FP-00612.
Dr. Mozaffarian reports research funding from the National Institutes of Health, the Gates Foundation, The Rockefeller Foundation, Vail Innovative Global Research, and the Kaiser Permanente Fund; personal fees from Acasti Pharma and Barilla; scientific advisory board, Beren Therapeutics, Brightseed, Calibrate, Elysium Health, Filtricine, HumanCo, Instacart Health, January Inc., and Perfect Day (ended: Day Two, Season Health, and Tiny Organics); stock ownership in Calibrate and HumanCo; and chapter royalties from UpToDate.
Footnotes
Conflicts of Interest. The remaining authors had no conflicts of interest to disclose.
Data Availability:
The CHS study makes phenotypic and genetic data available through the NIH public data repository BioLINCC (https://chs-nhlbi.org/CHS_PublicData).
References
- [1].Liu JJ, Green P, John Mann J, Rapoport SI, Sublette ME (2015) Pathways of polyunsaturated fatty acid utilization: Implications for brain function in neuropsychiatric health and disease. Brain Res. 1597, 220–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Crupi R, Marino A, Cuzzocrea S (2013) n-3 fatty acids: role in neurogenesis and neuroplasticity. Curr Med Chem 20, 2953–2963. [DOI] [PubMed] [Google Scholar]
- [3].Luchtman DW, Song C (2013) Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies. Neuropharmacology 64, 550–565. [DOI] [PubMed] [Google Scholar]
- [4].Gomez-Pinilla F (2008) Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 9, 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mensink RP, Katan MB (1992) Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arteriosclerosis & Thrombosis 12, 911–919. [DOI] [PubMed] [Google Scholar]
- [6].Imamura F, Micha R, Wu JH, de Oliveira Otto MC, Otite FO (2016) Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Medicine 13, e1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Caprari P, Scuteri A, Salvati AM, Bauco C, Cantafora A, Masella R, Modesti D, Tarzia A, Marigliano V (1999) Aging and red blood cell membrane: a study of centenarians. Exp Gerontol 34, 47–57. [DOI] [PubMed] [Google Scholar]
- [8].Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. (1991) The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1, 263–276. [DOI] [PubMed] [Google Scholar]
- [9].Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC (1999) Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. American Journal of Epidemiology 149, 531–540. [DOI] [PubMed] [Google Scholar]
- [10].Lai HT, de Oliveira Otto MC, Lemaitre RN, McKnight B, Song X, King IB, Chaves PH, Odden MC, Newman AB, Siscovick DS, Mozaffarian D (2018) Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: prospective cohort study. Bmj 363, k4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fretts AM, Imamura F, Marklund M, Micha R, Wu JHY, Murphy RA, Chien KL, McKnight B, Tintle N, Forouhi NG, Qureshi WT, Virtanen JK, Wong K, Wood AC, Lankinen M, Rajaobelina K, Harris TB, Djoussé L, Harris B, Wareham NJ, Steffen LM, Laakso M, Veenstra J, Samieri C, Brouwer IA, Yu CI, Koulman A, Steffen BT, Helmer C, Sotoodehnia N, Siscovick D, Gudnason V, Wagenknecht L, Voutilainen S, Tsai MY, Uusitupa M, Kalsbeek A, Berr C, Mozaffarian D, Lemaitre RN (2019) Associations of circulating very-long-chain saturated fatty acids and incident type 2 diabetes: a pooled analysis of prospective cohort studies. Am J Clin Nutr 109, 1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee Y, Nemet I, Wang Z, Lai HTM, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Sotoodehnia N, Budoff M, DiDonato JA, McKnight B, Tang WHW, Psaty BM, Siscovick DS, Hazen SL, Mozaffarian D (2021) Longitudinal Plasma Measures of Trimethylamine N-Oxide and Risk of Atherosclerotic Cardiovascular Disease Events in Community-Based Older Adults. J Am Heart Assoc 10, e020646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lemaitre RN, Jensen PN, Wang Z, Fretts AM, McKnight B, Nemet I, Biggs ML, Sotoodehnia N, de Oliveira Otto MC, Psaty BM, Siscovick DS, Hazen SL, Mozaffarian D (2021) Association of Trimethylamine N-Oxide and Related Metabolites in Plasma and Incident Type 2 Diabetes: The Cardiovascular Health Study. JAMA Netw Open 4, e2122844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang M, Wang Z, Lee Y, Lai HTM, de Oliveira Otto MC, Lemaitre RN, Fretts A, Sotoodehnia N, Budoff M, DiDonato JA, McKnight B, Tang WHW, Psaty BM, Siscovick DS, Hazen SL, Mozaffarian D (2022) Dietary Meat, Trimethylamine N-Oxide-Related Metabolites, and Incident Cardiovascular Disease Among Older Adults: The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 42, e273–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Willett WC, Stampfer M (1998) Issues in analysis and presentation of diatary data In Nutritional Epidemiology, Willet W, ed. Oxford University Press, New York, pp. 321–345. [Google Scholar]
- [16].Wu M, Ware JH (1979) On the use of repeated measurements in regression analysis with dichotomous responses. Biometrics 35, 513–521. [PubMed] [Google Scholar]
- [17].Willet W (1998) Nutritional Epidemiology, Oxford University Press, New York. [Google Scholar]
- [18].Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry 226, 497–509. [PubMed] [Google Scholar]
- [19].Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. Journal of lipid research 27, 114–120. [PubMed] [Google Scholar]
- [20].Taylor HL, Jacobs DR Jr., Schucker B, Knudsen J, Leon AS, Debacker G (1978) A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31, 741–755. [DOI] [PubMed] [Google Scholar]
- [21].Kumanyika S, Tell GS, Shemanski L, Polak J, Savage PJ (1994) Eating patterns of community-dwelling older adults: the Cardiovascular Health Study. Ann Epidemiol 4, 404–415. [DOI] [PubMed] [Google Scholar]
- [22].Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE (1985) Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122, 51–65. [DOI] [PubMed] [Google Scholar]
- [23].Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, Burke GL, Tracy R, Bhadelia R (1998) Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 29, 388–398. [DOI] [PubMed] [Google Scholar]
- [24].Arnold AM, Kronmal RA (2003) Multiple imputation of baseline data in the cardiovascular health study. Am J Epidemiol 157, 74–84. [DOI] [PubMed] [Google Scholar]
- [25].Teng EL, Chui HC (1987) The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 48, 314–318. [PubMed] [Google Scholar]
- [26].Arnold AM, Newman AB, Dermond N, Haan M, Fitzpatrick A (2009) Using telephone and informant assessments to estimate missing Modified Mini-Mental State Exam scores and rates of cognitive decline. The cardiovascular health study. Neuroepidemiology 33, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N (2003) Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 22, 1–12. [DOI] [PubMed] [Google Scholar]
- [28].Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JC, Jones B, Lyketsos C, Dulberg C (2004) Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 52, 195–204. [DOI] [PubMed] [Google Scholar]
- [29].McBee M (2010) Modeling Outcomes With Floor or Ceiling Effects: An Introduction to the Tobit Model. Gifted Child Quarterly 54, 314–320. [Google Scholar]
- [30].Elkins JS, Longstreth WT Jr., Manolio TA, Newman AB, Bhadelia RA, Johnston SC (2006) Education and the cognitive decline associated with MRI-defined brain infarct. Neurology 67, 435–440. [DOI] [PubMed] [Google Scholar]
- [31].Fitzmaurice GML NM; Ware JH (2011) Modeling the Mean: Analyzing Response Profiles In Applied Longitudinal Analysis John Wiley & Sons Inc., Hoboken, New Jersey, pp. 105–140. [Google Scholar]
- [32].Allison PD (2010) Survival Analysis Using SAS: A Practical Guide, SAS Institute Inc., North Carolina. [Google Scholar]
- [33].Allison PD (2010) Estimating Cox Regression Models with PROC PHREG In Survival Analysis Using SAS: A Practical Guide SAS Institute, Cary, NC, pp. 125–201. [Google Scholar]
- [34].Tallima H, El Ridi R (2018) Arachidonic acid: Physiological roles and potential health benefits - A review. J Adv Res 11, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang X, Sjogren P, Cederholm T, Arnlov J, Lindholm B, Riserus U, Carrero JJ (2014) Serum and adipose tissue fatty acid composition as biomarkers of habitual dietary fat intake in elderly men with chronic kidney disease. Nephrol Dial Transplant 29, 128–136. [DOI] [PubMed] [Google Scholar]
- [36].de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR Jr., Mozaffarian D (2013) Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2, e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wu JH, Lemaitre RN, King IB, Song X, Psaty BM, Siscovick DS, Mozaffarian D (2014) Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: the Cardiovascular Health Study. Circulation 130, 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Assmann KE, Adjibade M, Hercberg S, Galan P, Kesse-Guyot E (2018) Unsaturated Fatty Acid Intakes During Midlife Are Positively Associated with Later Cognitive Function in Older Adults with Modulating Effects of Antioxidant Supplementation. J Nutr 148, 1938–1945. [DOI] [PubMed] [Google Scholar]
- [39].Keim SA, Boone KM, Klebanoff MA, Turner AN, Rausch J, Nelin MA, Rogers LK, Yeates KO, Nelin L, Sheppard KW (2018) Effect of Docosahexaenoic Acid Supplementation vs Placebo on Developmental Outcomes of Toddlers Born Preterm: A Randomized Clinical Trial. JAMA Pediatr 172, 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Henjum S, Kvestad I, Shrestha M, Ulak M, Chandyo RK, Thorne-Lyman AL, Shrestha PS, Kjellevold M, Hysing M, Strand TA (2018) Erythrocyte DHA and AA in infancy is not associated with developmental status and cognitive functioning five years later in Nepalese children. Nutr J 17, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Crozier SR, Sibbons CM, Fisk HL, Godfrey KM, Calder PC (2018) Arachidonic acid and DHA status in pregnant women is not associated with cognitive performance of their children at 4 or 6–7 years. 119, 1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chandrasekharan JA, Sharma-Walia N (2015) Lipoxins: nature’s way to resolve inflammation. J Inflamm Res 8, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mazereeuw G, Lanctot KL, Chau SA, Swardfager W, Herrmann N (2012) Effects of omega-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol Aging 33, 1482 e1417–1429. [DOI] [PubMed] [Google Scholar]
- [45].Oulhaj A, Jerneren F, Refsum H, Smith AD, de Jager CA (2016) Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J Alzheimers Dis 50, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jerneren F, Elshorbagy AK, Oulhaj A, Smith SM, Refsum H, Smith AD (2015) Brain atrophy in cognitively impaired elderly: the importance of long-chain omega-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr 102, 215–221. [DOI] [PubMed] [Google Scholar]
- [47].Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA (2006) Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol 63, 1545–1550. [DOI] [PubMed] [Google Scholar]
- [48].Samieri C, Feart C, Letenneur L, Dartigues JF, Peres K, Auriacombe S, Peuchant E, Delcourt C, Barberger-Gateau P (2008) Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr 88, 714–721. [DOI] [PubMed] [Google Scholar]
- [49].Kroger E, Verreault R, Carmichael PH, Lindsay J, Julien P, Dewailly E, Ayotte P, Laurin D (2009) Omega-3 fatty acids and risk of dementia: the Canadian Study of Health and Aging. Am J Clin Nutr 90, 184–192. [DOI] [PubMed] [Google Scholar]
- [50].Laurin D, Verreault R, Lindsay J, Dewailly E, Holub BJ (2003) Omega-3 fatty acids and risk of cognitive impairment and dementia. J Alzheimers Dis 5, 315–322. [DOI] [PubMed] [Google Scholar]
- [51].Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR (2007) Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr 85, 1103–1111. [DOI] [PubMed] [Google Scholar]
- [52].Igarashi M, Kim HW, Chang L, Ma K, Rapoport SI (2012) Dietary n-6 polyunsaturated fatty acid deprivation increases docosahexaenoic acid metabolism in rat brain. J Neurochem 120, 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fernandes MF, Mutch DM, Leri F (2017) The Relationship between Fatty Acids and Different Depression-Related Brain Regions, and Their Potential Role as Biomarkers of Response to Antidepressants. Nutrients 9(3):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The CHS study makes phenotypic and genetic data available through the NIH public data repository BioLINCC (https://chs-nhlbi.org/CHS_PublicData).