Figure 4: Ser129P regulates association of α-syn with functional binding partners VAMP2 and synapsin.
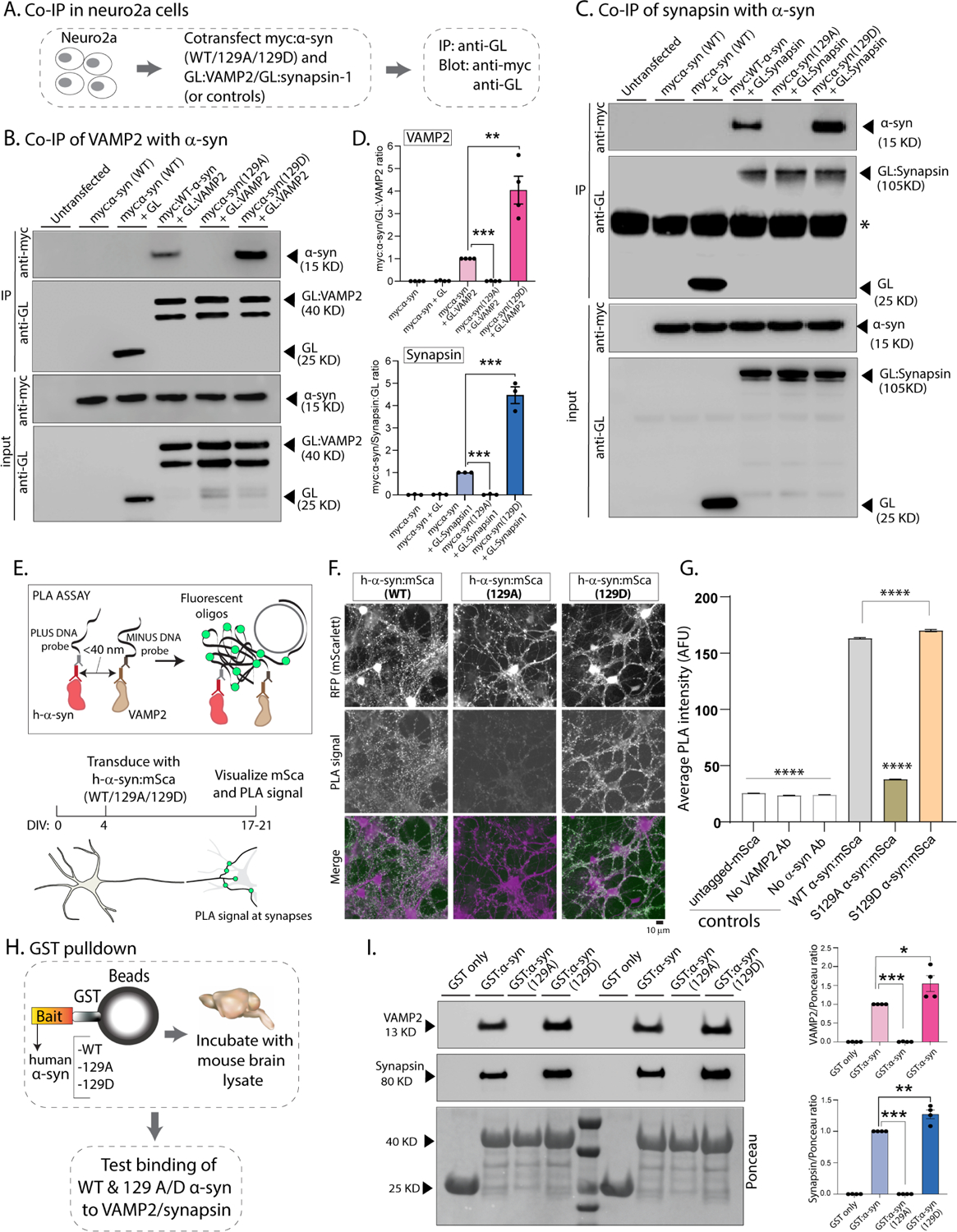
(A) Workflow for co-immunoprecipitation experiments in neuro2a cells.
(B, C) Western blots from co-immunoprecipitation experiments show that both VAMP2 (B) and synapsin (C) co-immunoprecipitated with WT-h-α-syn but not phospho-incompetent (129A) h-α-syn. Mimicking Ser129P (S129D) augmented this interaction – quantified on the right (n=4 for myc α-syn/GL-VAMP2 and n=3 for myc α-syn/GL-Synapsin co-IP). All western-blot data quantified in (D, mean ± SEM ***p<0.0001, unpaired Student’s t-test).
(E) Principle of our PLA assay and experimental design (neuron cartoon courtesy of Christophe Leterrier, Marseille). Note that a fluorescent signal is expected if transduced h-α-syn:mScarlet (WT or 129A/D) and endogenous mouse VAMP2 are <40 nm apart.
(F) Representative images of fluorescent mScarlet and PLA signals. Note that neurons transduced with WT h-α-syn show punctate PLA signal at synapses (left column), while essentially no signal is seen with phospho-incompetent (S129A) h-α-syn (middle column). Increased PLA-signal is seen with S129D h-α-syn (right column); all data quantified in (G, mean ± SEM ****p<0.0001 one-way ANOVA followed by a nonparametric Kruskal-Wallis test).
(H) Workflow for pulldown of GST-tagged WT/129A/129D h-α-syn after incubation with mouse brain lysates. Equivalent amounts of immobilized GST h-α-syn (or its phospho variants) were used.
(I) Samples from GST-pulldown were analyzed by NuPAGE and immunoblotted with antibodies against VAMP2 (top panel) and synapsin (middle panel); two biological replicates are shown. Ponceau staining (bottom panel) shows equivalent loading of fusion proteins. Note that preventing Ser129P (S129A) blocked interaction with VAMP2 and synapsin; blots quantified below (n=4, mean ± SEM *p<0.01,**p<0.001,***p<0.0001, unpaired Student’s t-test). Also note that the GST-h-α-syn in our experiments appears to be innately phosphorylated at Ser129 (see results and further characterization in Supp. Fig. 5).
