Fig. 2.
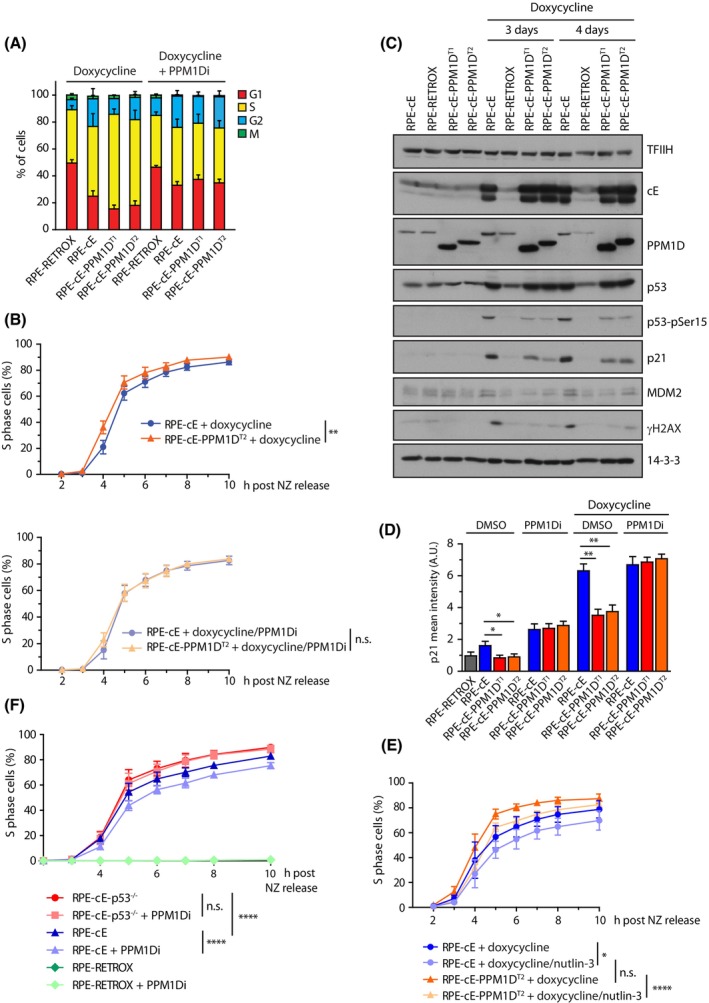
PPM1D accelerates G1/S transition and prolonges S phase upon cyclin E1 induction. (A) Parental RPE1‐Retrox, RPE1‐cE and two clones RPE1‐cE‐PPM1DT cells were treated either with doxycycline or a combination of doxycycline and PPM1Di for 4 days and cells were incubated with EdU 30 min prior harvesting. After fixation, cells were probed with Mpm2 antibody, EdU was stained using click‐it reaction, DNA content with DAPI and cells were analysed by flow cytometry. Cell cycle phases were determined as 2n EdU− for G1, EdU+ for S, 4n EdU− for G2 and 4n, Mpm2+ for M. Bars indicate SD (n = 4). (B) RPE1‐cE and RPE1‐cE‐PPM1DT2 cells were synchronized in mitosis by nocodazole and were released into fresh media containing EdU and supplemented with doxycycline or combination of doxycycline and PPM1Di. Cells were collected at indicated time intervals and entry to S phase was monitored using flow cytometry as a fraction of EdU+ cells (n = 4). Samples were analysed in parallel but for clarity, they are presented in two separate graphs. Bars indicate SD. Statistical significance was determined by two‐way ANOVA (n.s., nonsignificant P > 0.05, **P < 0.01). (C) RPE1‐cE, RPE1‐cE‐PPM1DT1 and RPE1‐cE‐PPM1DT2 cells were treated with doxycycline for 3 or 4 days and whole cell lysates were analysed by immunoblotting. Representative result from two independent experiments is shown. (D) RPE1‐Retrox, RPE1‐cE, RPE1‐cE‐PPM1DT1 and RPE1‐cE‐PPM1DT2 cells were treated or not with doxycycline, PPM1Di and combination of both for 4 days. After fixation, cells were stained with p21 antibody and imaged using ScanR. Plotted is the mean nuclear intensity. More than 1000 cells were quantified per experiment, n = 3. Statistical significance was evaluated by the two‐tailed t‐test, error bars indicate SDs (n = 3) (**P < 0.01; *P ≤ 0.05). (E) Cells were synchronized an analysed as in B. Where indicated, nutlin‐3 (9 μm) was added to the media at time of release from the nocodazole block. Statistical significance was determined by two‐way ANOVA (n = 3; n.s., nonsignificant P > 0.05; *P ≤ 0.05, ****P ≤ 0.0001). (F) RPE1‐cE and RPE1‐cE‐p53−/− cells were synchronized in mitosis by nocodazole and were released into fresh media containing EdU and supplemented with doxycycline or combination of doxycycline and PPM1Di. Entry to S phase was monitored using flow cytometry as a fraction of EdU+ cells (n = 4). Statistical significance was determined by two‐way ANOVA (****P ≤ 0.0001).
