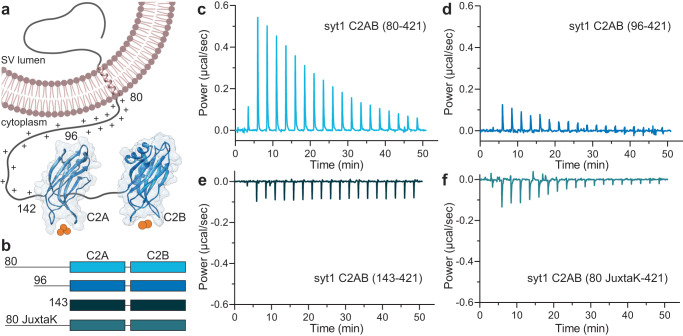
Fig. 1. The juxtamembrane linker reduces the Ca2+ affinity of the C2-domains of syt1.
a Depiction of full-length syt1 embedded in a synaptic vesicle membrane. The juxtamembrane segment, residues 80–142, contains nineteen lysine residues (indicated by the +), concentrated within residues 80–95. The Ca2+-binding C2-domains, C2A and C2B, were rendered using UCSF Chimera and PDB files 1RSY and 1K5W; Ca2+ ions are shown as orange spheres. The synaptic vesicle membrane was created using BioRender. b Schematic diagram of the syt1 C2AB constructs used for isothermal titration calorimetry (ITC), which includes the entire juxtamembrane linker (80–142), a truncated linker starting at position 96 (extensively used in the literature), complete removal of the linker, and a mutated JuxtaK linker (80 JuxtaK) in which the lysine residues have been substituted to other polar residues39. c–f Representative ITC traces showing the heat of Ca2+ binding to each of the constructs shown in b; n = 3. The linker reduces the affinity of C2-domains for Ca2+. This effect is largely abrogated in the JuxtaK mutant; moreover, Ca2+ binding became exothermic for this mutant linker. Dissociation constants and thermodynamic values are reported in Table 1 and Supplementary Table 1, respectively.