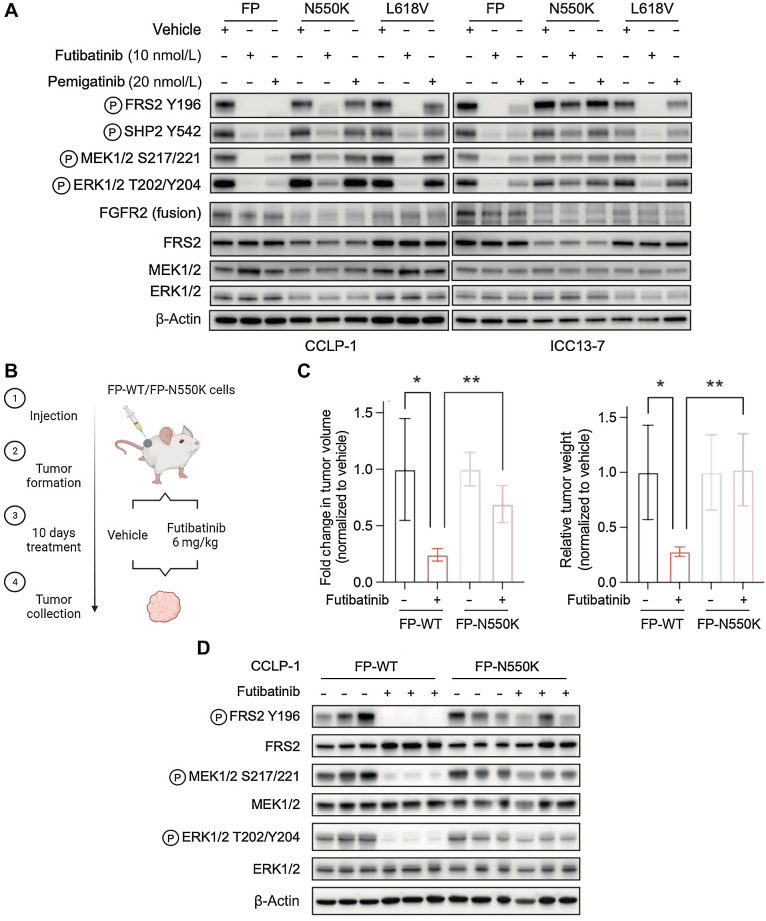
Figure 3.
The recurrent molecular brake mutation, N550K, drives resistance to clinically achievable pan-FGFR TKI dose levels. A, Immunoblot analysis of signaling proteins in CCLP-1 or ICC13–7 cells engineered to express the FGFR2–PHGDH fusion protein with wild-type kinase domain or with the N550K or L618V mutations. Cells were treated with vehicle, 10 nmol/L futibatinib, or 20 nmol/L pemigatinib for 4 hours. B–D,In vivo assessment of futibatinib efficacy against the FGFR2 molecular brake mutation, N550K. B, Schematic diagram of experiment design. Mice harboring FGFR-dependent CCLP-1 xenografts expressing the indicated FGFR2-PHGDH fusion (FP) with a WT kinase domain or with the N550K mutation were treated with vehicle (n = 4) or futibatinib 6 mg/kg (n = 4) daily for 10 days. Treatment was started once tumors reached a volume approximately 200 mm3. C, Relative fold change of tumor volume (left) or tumor weight (right) compared with vehicle treatment at the end point. **, P < 0.01. Data are shown as mean ± SD. D, Immunoblot analysis of tumor lysates. Tumors were harvested 4 hours after the last dose of treatment. (B, Created with BioRender.com.)