Figure 1.
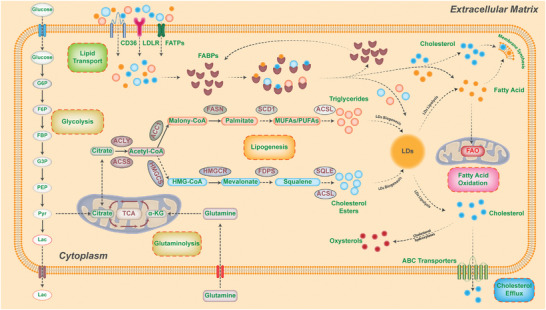
Rewiring of lipid metabolism in cancer. Lipid metabolism is a dynamic biological process that involves the endogenous de novo synthesis, exogenous import of fatty acids and cholesterol, fatty acid β oxidation, cholesterol efflux, biogenesis, and lipolysis of lipid droplets. Intracellular de novo lipogenesis begins with acetyl‐coenzyme A (acetyl‐CoA) derived from acetate by ATP‐citrate lyase (ACLY) or citrate by acetyl‐CoA synthetase (ACSS). Fatty acid synthesis requires acetyl‐CoA carboxylation into malonyl‐CoA by acetyl‐CoA carboxylases (ACC1/2), followed by the condensation of seven malonyl‐CoA molecules and one acetyl‐CoA molecule into the saturated 16‐carbon palmitate (16:0) by fatty acid synthase (FASN). Palmitate is then desaturated by stearoyl‐CoA desaturases (SCD) or elongated by fatty acid elongases (ELOVL) to form the monounsaturated 16‐carbon palmitoleate (16:1 n‐7) or 18‐carbon oleate (18:1 n‐9). Biogenesis of cholesterol also begins with acetyl‐CoA via the mevalonate pathway, which results in the synthesis of squalene and finally, cholesterol. Cancer cells can acquire fatty acids and cholesterol from various extracellular sources, such as LDL particles or fatty acid transport proteins. When lipids accumulate, cancer cells use these lipids to meet their energy consumption demand and redox homeostasis through fatty acid oxidation or β‐oxidation. Excess cholesterol is exported to the blood or converted into oxysterols through oxidation processes. Surplus fatty acids are esterified with glycerol or cholesterol into triglycerides and cholesteryl esters, which are incorporated into lipid droplets (LDs). When energy or membrane synthesis is needed, lipid droplets can be rapidly lipolyzed into free fatty acids and cholesterols to facilitate cancer cell proliferation and progression.
