Figure 3.
Determining stem cell potential in dividing and quiescent cells
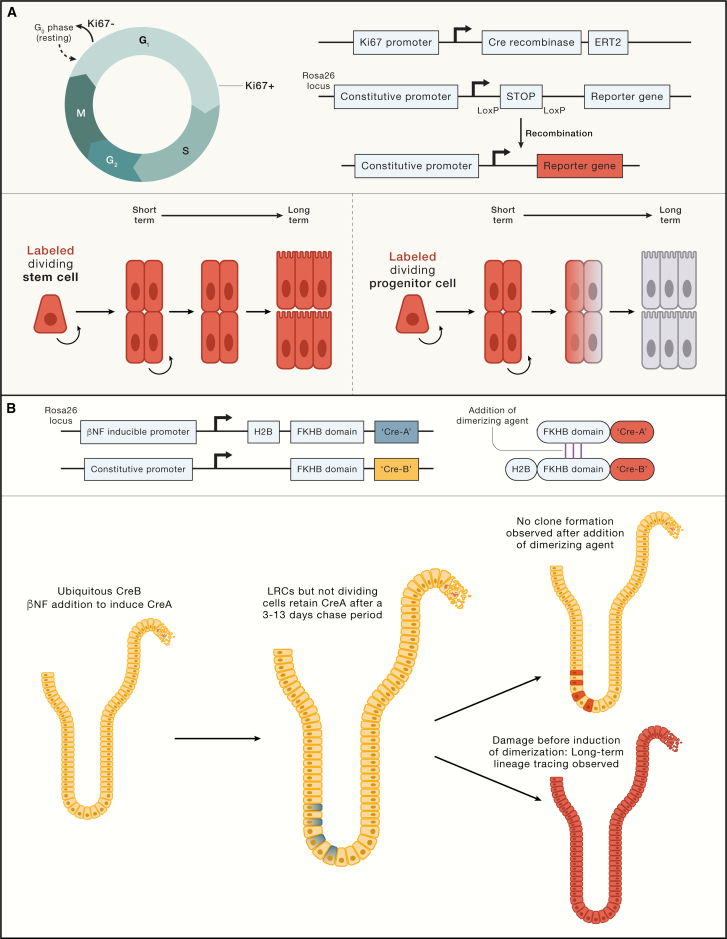
(A) Proliferating cells can be traced exploiting expression of KI67. KI67 is restricted to all cell-cycle phases except G0. A KI67-expression controlled Cre-recombinase can be used to trace dividing cells to assess their long-term clonogenicity and multipotency. When reporter recombination is initiated in a dividing stem cell (labeled in red, first scenario), long-term tracing will be observed. When the dividing cell is a committed progenitor, labeling is transient and will eventually be lost (second scenario).
(B) Strategy to perform lineage tracing from slowly dividing or post-mitotic label-retaining cells (LRCs) in the murine small intestine. Cre-recombinase is expressed as two parts: the ubiquitously produced C-terminal CreB (in yellow) and the N-terminal CreA that is fused to histone 2B (H2B) (in blue). CreA expression can be induced by the addition of β-naphthoflavone (βNF), followed by cell-cycle-dependent dilution of the H2B-CreA protein. Both Cre-halves are additionally fused to an FKHB domain, of which fusion can be induced by a small molecule in order to reconstitute a functional enzyme and induce lineage tracing (traced cells will be red).
CreB is constitutively expressed (yellow cells, left). Induction of CreA expression by βNF followed by a 3–13 days dilution will lead to a scenario in which only slowly dividing LRCs and post-mitotic cells retain both parts of the enzyme (blue and yellow cells, middle). The induction of dimerization does not yield long-term clone formation, suggesting that slowly dividing cells are not homeostatic stem cells (red cells, top). When epithelial damage is induced before dimerization of the Cre-domains, long-term tracing can be observed (bottom). These experiments evidence that LRCs are not homeostatic stem cells, but regain stemness upon damage.