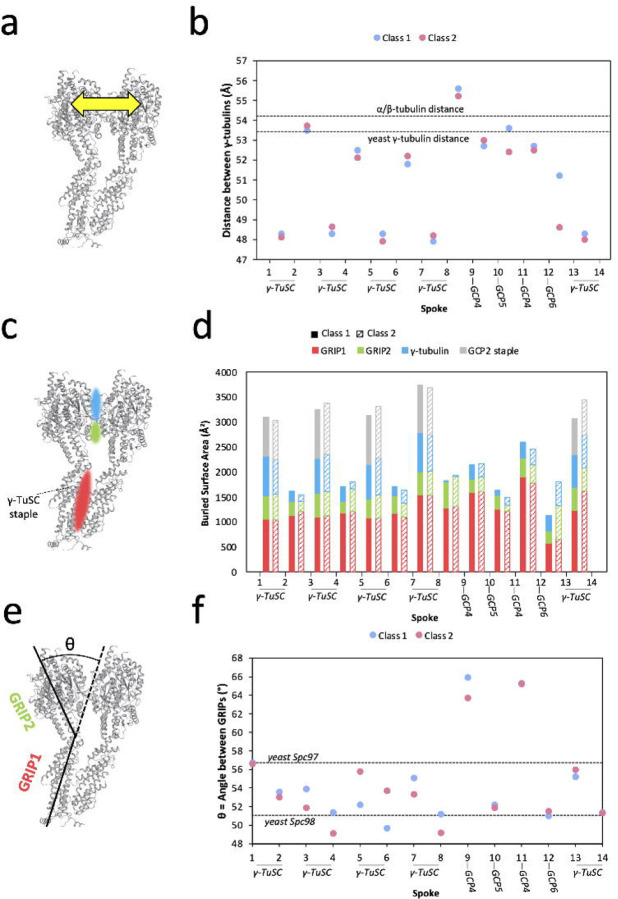
Figure 3: Local asymmetry of γ-TuRC leads to template imperfection.
a. Schematic for measuring distances between adjacent γ-tubulin subunits. Distances were measured from the centroid residue Tyr-169.
b. Measurement of distances between adjacent γ-tubulin subunits. Distances for Class 1 are shown in blue and distance for Class 2 in pink. Measurements were calculated as schematized in (A). Dashed lines indicate reference distances between adjacent γ-tubulin subunits in the closed yeast γ-TuRC (PDB accession 7M2W), measured between the γ-tubulin centroid residue Tyr-170 as 53.6 Å around the symmetric helix, and between adjacent β-tubulin subunits in the 13 protofilament MT (PDB accession 7SJ7), measured between the β-tubulin centroid residue Phe-167 as 54.2 Å around the symmetric helix.
c. Schematic for measuring buried surface areas between adjacent γ-TuRC spokes. Colored patches represent contact areas where buried surface area is measured. Red represents RIP1, the N-terminal half of the GCP, green represents GRIP2, the C-terminal half of the GCP, and blue represents γ-tubulin.
d. Measurement of buried surface area between adjacent γ-TuRC spokes. Buried surface area is tabulated for GRIP1 (red), GRIP2 (green), and γ-tubulin (blue). When the contact is between GCP2 and GCP3 within a γ-TuSC, the surface area buried by the staple is also tabulated. Surface areas for Class 1 are shown as solid bars and, for Class 2, as striped bars. The precise residues corresponding to GRIP domain assignments can be found in the Methods section.
e. Schematic for measuring the angle between the GRIP1 and GRIP2 domains of GCP subunits.
f. Measurements of the angle between GRIP1 and GRIP2 domains for each spoke of Class 1 (blue) and Class 2 (pink). Dashed lines show the angle displayed for yeast Spc97 (analogous to GCP2, 56.4°, and yeast Spc98 (analogous to GCP3, 51° in the closed yeast γ-TuRC (PDB accession 7M2W). Residues used to calculate domain angles can be found in the Methods section.