Abstract
To ensure that the embryo can package exponentially increasing amounts of DNA, replication-dependent histones are some of the earliest transcribed genes from the zygotic genome. However, how the histone genes are identified is not known. The pioneer factors Zelda and CLAMP collaborate at a subset of genes to regulate zygotic genome activation in Drosophila melanogaster and target early activated genes to induce transcription. CLAMP also regulates the embryonic histone genes and helps establish the histone locus body, a suite of factors that controls histone mRNA biosynthesis. The relationship between Zelda and CLAMP led us to hypothesize that Zelda helps identify histone genes for early embryonic expression. We found that Zelda targets the histone locus early during embryogenesis, prior to histone gene expression. However, depletion of zelda in the early embryo does not affect histone mRNA levels or histone locus body formation. While surprising, these results concur with other investigations into Zelda’s role in the early embryo, suggesting the earliest factors responsible for specifying the zygotic histone genes remain undiscovered.
Introduction
To efficiently store nearly 2 meters of DNA in a nucleus of only ~10 micrometers, eukaryotic cells utilize histone-mediated compaction and organization of DNA through the use of nucleosomes, histone protein octamers consisting of two dimers of H2A-H2B and two dimers of H3-H4, a linker histone H1, and the associated ~150bp of DNA (Annunziato, 2008). The canonical, replication-dependent histones often exist as a multi-gene family with dozens or hundreds of coding sequences (Bongartz & Schloissnig, 2019; Marzluff et al., 2002). The many copies of the histone genes may cluster, but the number of distinct histone loci varies across species with 2 to 4 in most mammals (Seal et al., 2022), 11 in C. elegans (Roberts et al., 1987), and 1 in Drosophila melanogaster (White et al., 2011). This clustered organization ensures that histone genes are efficiently and quickly expressed to organize the newly synthesized DNA during DNA replication, prior to nuclear/cellular division (Mei et al., 2017; Romeo & Schümperli, 2016). Disruption of proper histone gene expression such that too few or too many histones are produced can have disastrous effects: in the rapidly-dividing developing metazoan embryo, where histones are especially important, a reduction of histones slows cell divisions, whereas histone overexpression causes aberrant divisions and perturbs cell cycle timing. Both conditions lead to embryonic lethality (Amodeo et al., 2015; Chari et al., 2019).
Metazoan embryogenesis represents a period of rapid nuclear/cellular divisions, as quickly as every 8 minutes in the fruit fly (McCleland, Shermoen, & O’Farrell, 2009). Initially, to accommodate the doubling of DNA every cycle, the egg is maternally loaded with vast amounts of histone mRNA that are translationally upregulated after fertilization (Horard & Loppin, 2015; Li et al., 2012). This maternal deposition allows the zygote to proceed through development without the burden of producing its own histone transcripts. Eventually, however, when the maternal transcripts are exhausted or degraded, the zygotic genome must take over transcriptional responsibility (Tadros & Lipshitz, 2009). This process of an embryo beginning to transcribe its own genome is called Zygotic Genome Activation (ZGA) and is not specific to the histone genes, but targets early developmental genes (Schulz & Harrison, 2019).
The minor, early wave of ZGA in D. melanogaster begins at nuclear cycle 8 and concludes at cycle 12 when the major, later wave begins and the remainder of the genome is activated (De Renzis et al., 2007; Tadros & Lipshitz, 2009). Zygotic histone gene expression is first detectable around nuclear cycle 11 (White et al., 2007). Zygotic histone expression is accompanied by the formation of a phase-separated, nuclear body consisting of a suite of factors responsible for controlling histone mRNA biosynthesis and mRNA processing called the Histone Locus Body (HLB) (Liu et al., 2006; Tatomer et al., 2016). Although some members of the HLB are known and a general understanding of its basic structure exists (Kemp et al., 2021; Salzler et al., 2013), it is unclear how the histone genes are targeted so early during embryogenesis for their unique regulation, as no HLB specific factors interact with DNA sequence.
The Drosophila pioneer factor CLAMP binds genome wide (Larschan et al., 2012), but is also important for HLB formation and proper embryonic expression of the histone genes (Rieder et al., 2017). CLAMP is currently the only known Drosophila HLB member, aside from general transcription factors, with DNA binding capability (Tatomer et al., 2016): it recognizes GA-repeat cis elements present in the histone3/histone4 promoter (Koreski et al., 2020; Rieder et al., 2017). Elsewhere, CLAMP functions with another pioneer factor, Zelda, to control ZGA across the fly genome (Duan et al., 2021). Zelda is considered the “master regulator” of Drosophila ZGA, binding early, immediately prior to the minor wave of ZGA (Hamm & Harrison, 2018; Harrison et al., 2011). Zelda’s role at the histone locus is unknown, but prior research revealed an intriguing connection: maternal germline zelda RNAi resulted in larger histone3 RNA FISH and HLB factor puncta in the early embryo (Huang et al., 2021). The above observations led us to hypothesize that Zelda helps to specify the histone genes prior to widespread ZGA, plays a role in HLB formation, and regulates zygotic histone biogenesis.
Here, we demonstrate that Zelda targets several sites in the histone gene array prior to zygotic histone gene expression. However, modulating Zelda’s presence at the histone locus has little effect on histone mRNA levels in the early embryo. Zelda depletion by RNAi does not cause detectable changes in HLB formation or histone mRNA levels. Additionally, elimination of Zelda’s DNA binding sites within a transgenic histone gene array does not prevent HLB factor recruitment to the transgene. Overall, we conclude that Zelda has a dispensable role in HLB formation and histone gene regulation in the early Drosophila embryo. This finding is surprising given Zelda’s status as the master regulator of ZGA and collaboration with CLAMP. The mechanism for specific, early activation of the histone genes remains undiscovered.
Results and Discussion
Zelda localizes to TAGteam sites in the histone gene array early during embryogenesis
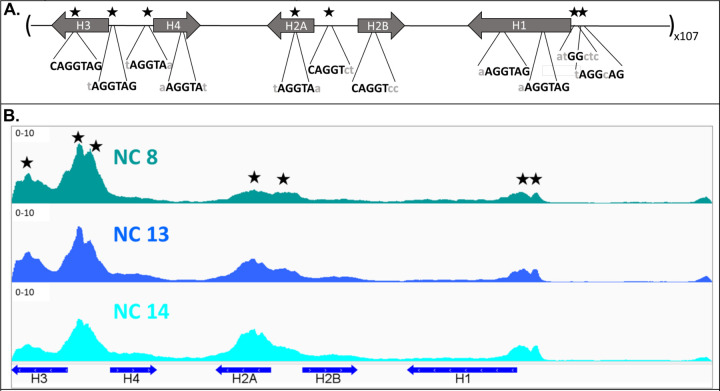
Zelda recognizes and binds DNA via a series of C2H2 zinc fingers near its C-terminus(McDaniel et al., 2019). Zelda targets TAGteam sites, which include the seven base pair motif: CAGGTAG(Rahul Satija & Robert K. Bradley, 2012). We identified several likely TAGteam sites within the ~5 Kb D. melanogaster histone gene array. The sequences are present in the promoters and gene bodies of the canonical histone genes (Fig. 1A).
Figure 1. Zelda targets TAGteam sites in the Drosophila early embryonic histone gene array.
(A) The Drosophila melanogaster histone locus contains ~100 tandem repeats of a 5-kb array containing the five canonical replication-dependent histone genes. Derivatives of the TAGteam sequence recognized by the Zelda protein, CAGGTAG, are present across the locus. Seven of these sites correspond to Zelda ChIP-seq signal peaks (Harrison et al., 2011) and are denoted by stars. (B) Zelda ChIP-seq shows that Zelda targets the histone gene array as early as nuclear cycle (NC) 8 (teal tracks), immediately after Zelda is translationally upregulated and the start of the minor wave of ZGA. Zelda remains at the array through ZGA, though its presence shifts from predominately at the histone3/histone4 promoters to the evenly distributed at the former and the histone2a gene body (dark blue and light blue tracks).
We began by mapping previously published Zelda ChIP-seq data from early, staged Drosophila embryos(Harrison et al., 2011) to the histone gene array. Because the ~100 histone gene arrays are nearly identical in sequence(Bongartz & Schloissnig, 2019), we can collapse all ChIP-seq data onto a single histone array(McKay et al., 2015). We discovered that Zelda targets the histone gene array by embryonic nuclear cycle 8 (Fig. 1B), the beginning of the minor wave of ZGA and immediately after Zelda is translationally upregulated(McDaniel et al., 2019). Zelda recognizes 7 sites in the array, which correspond to predicted TAGteam motifs (Fig. 1A) and targets the histone gene array prior to histone gene expression and HLB factor localization, which is first detectable around nuclear cycle 11(Edgar & Schubiger, 1986; White et al., 2011). Initially, Zelda ChIP signal is highest over sites in the histone3/histone4 promoter. This promoter is the minimal sequence required for HLB formation and contains important cis-regulatory elements targeted by CLAMP(Koreski et al., 2020; Rieder et al., 2017; Salzler et al., 2013). Zelda’s relative distribution across the array changes as development proceeds, though the significance of this distribution, if any, is unclear.
Zelda reduction in the embryo has little effect on histone transcript levels and HLB factor recruitment
To investigate Zelda’s role in histone gene regulation in the early embryo, we began with an RNAi-mediated depletion (Ni et al., 2011)of maternally deposited zelda (Yamada et al., 2019)and measured the effect on histone transcript levels. Using the “maternal triple” GAL4 driver (MTD), we expressed zelda shRNA in adult female ovaries and depleted maternally deposited zelda mRNA in the egg and early embryo. As previously observed, maternal germline zelda RNAi led to nearly 100% embryonic lethality (Duan et al., 2021). Premature Zelda translation does not result in premature gene activation, but alters the levels of transcripts at the normal time of activation (Larson et al., 2022).
When quantified through qPCR, we found that zelda RNAi led to significantly reduced levels of zelda in 2–4 hour embryos (2–4 hour post-lay), less than 10% of control mCherry RNAi levels (Fig. 2A). However, zelda depletion did not result in meaningful changes in histone mRNA levels in post-ZGA embryos. Although, we did observe a slight increase in histone4 mRNA levels. Our observations regarding zelda RNAi are very different from our published observations regarding clamp RNAi (Rieder et al., 2017), which gives a similarly striking reduction of clamp in the early embryo but also leads to significantly decreased levels of multiple histone mRNA levels in post ZGA embryos (Fig. 2A). We also observe the documented upregulation of zelda/clamp levels in the embryo when the reciprocal factor is depleted (Duan et al., 2021), serving as additional confirmation of RNAi efficacy.
Figure 2. Depletion of zelda in the early embryo does not significantly affect histone mRNA levels or HLB factor recruitment.
(A)We performed qRT-PCR for histone and transcription factor mRNA in 2–4 hour post lay (after the start of ZGA and zygotic histone gene expression) embryos under control (mCherry RNAi), Zelda depleted (zelda RNAi), and CLAMP depleted (clamp RNAi) conditions. We observe little to no change in histone mRNA levels in the zelda depleted embryos compared to controls outside of a slight increase in histone4 mRNA levels (* denotes a p-value≤0.05, Student’s t-test). In contrast, we see significantly decreased histone transcripts in our clamp depleted conditions compared to controls (* denotes a p-value≤0.05, Student’s t-test). Error bars represent ± standard error. Expression is normalized to rp49. (B) We performed immunostaining for the HLB component, Mxc, in early embryos in control (mCherry) and zelda depleted conditions. We observed no difference in Mxc. (C) We utilized a GFP-tagged Zelda observe potential localization of Zelda in embryos. We did not see clear colocalization between Mxc and Zelda in control (mCherry) or zelda depleted conditions, in contrast to previous results with CLAMP (Rieder et al., 2017).
Although zelda depletion does not greatly affect histone transcript levels, we reasoned that it might affect HLB formation. We therefore performed early embryo immunostaining for Mxc, a core scaffolding HLB factor that is one of the first present at the locus (Terzo et al., 2015; White et al., 2011), to detect body formation under zelda depletion conditions. The HLB is visible as either 1 or 2 Mxc puncta in wild-type zygotic nuclei, depending on whether homologous chromosomes are paired or unpaired (Fig. 2B). Zelda RNAi embryos die around ZGA but progress far enough through development that we can perform immunostaining to detect HLB formation. We observe no change in HLB puncta formation with regards to presence and qualitative puncta size in zelda RNAi embryos, compared to control mCherry RNAi embryos (Fig. 2B). Our observations contrast with previously published observations that histone3 RNA FISH and Mxc puncta increase in diameter upon zelda RNAi (Huang et al., 2021).
Zelda therefore appears to be dispensable for zygotic histone gene expression and HLB formation in the embryo, despite targeting the histone gene array prior to HLB formation. We were surprised by these conclusions, given that Zelda regulates key embryonic genes during ZGA. However, Zelda is implicated in multiple regulatory pathways in the early embryo, including the establishment of three-dimensional genome organization (Hug et al., 2017). Loss of Zelda during development leads to loss of insulation in a locus-specific manner. It is possible that Zelda has a role in organizing the histone locus and does not directly affect transcription of the histone genes or recruitment of HLB factors. This scenario could explain the published observations by Huang et al., who observed increased Mxc puncta size after zelda depletion. However, we did not replicate this phenomenon in our RNAi experiments. Conversely, it may be more likely that the continued presence and increased size of HLBs in zelda depletion embryos are because other Zelda-dependent regions of the genome remain inactivated. Additional support for this model comes from a recent study that demonstrates that zelda depletion affects RNA Polymerase II clustering across the nucleus, except at the replication-dependent histone genes (Cho et al., 2022).
Zelda and CLAMP do not affect reciprocal localization to the histone genes
We previously demonstrated that CLAMP targets the histone3/histone4 promoter by embryonic nuclear cycle 10 (Rieder et al., 2017). Zelda and CLAMP bind targets across the genome during ZGA and regulate each other’s binding at a subset of promoters genome-wide. The binding motifs of Zelda and CLAMP (TAGteam sites and GA-repeats, respectively) are often found in close proximity, within 2kb, across the genome (Duan et al., 2021). We previously performed zelda and clamp RNAi in early embryos and reciprocal ChIP-seq for the other factor. We mapped these early embryo ChIP-seq datasets to the histone gene array to discover if these two pioneer factors target the locus in collaboration. Upon zelda RNAi, CLAMP localization to the histone array remains relatively unchanged with a strong peak at the histone3/histone4 promoter. Under clamp RNAi conditions, CLAMP binding at the locus decreases and changes its distribution across the locus. Under zelda RNAi conditions, Zelda is largely lost at the genes, although some small amount appears to remain. Upon clamp RNAi, Zelda’s localization to the locus remains mostly unchanged, however, there is a slight drop in binding near the histone1 gene (Fig. S1). Overall, the loss of one factor does not greatly affect the binding of the other at the histone genes, which conflicts with the relationship elsewhere in the genome where the two play a reciprocal role in the other’s localization and activity(Duan et al., 2021).
We previously documented that even though maternal clamp RNAi reduced CLAMP to undetectable levels in the early embryo by western blot, small amounts of maternal CLAMP are still visible, specifically at the histone locus, by immunostaining in the early embryo (Rieder et al., 2017). We therefore hypothesized that a small amount of Zelda might also be detectable at the zygotic histone locus by immunofluorescence. We performed post-ZGA embryo immunofluorescence for Zelda using an endogenously CRISPR-tagged Zelda-GFP (D. C. Hamm et al., 2017) under mCherry and zelda RNAi conditions. Although we confirmed effective zelda knock-down by qPCR (Fig. 2A), we did not observe enrichment of Zelda-GFP signal specifically overlapping with Mxc in either condition; Zelda is broadly nuclear under control (mCherry RNAi) conditions, and the majority of this nuclear signal is lost after zelda depletion (Fig. 2C). Although we do not detect Zelda at the histone locus by immunofluorescence, we did observe traces of Zelda through ChIP-seq after zelda RNAi (Fig. S1), indicating that small amounts of Zelda may remain at the locus even after depletion.
Regulation of the histone genes and the production of sufficient histone proteins is an exceptionally important process, especially in a rapidly dividing/replicating embryo, so nuclei may implement strategies to increase localization and retention of imperative factors. Our findings may explain why zelda depletion has little effect on histone transcript levels, as a small amount of Zelda that escapes RNAi may still localize to the histone genes and be sufficient for inducing gene expression. However, we do not observe Zelda colocalizing with Mxc even under control conditions, in contrast to CLAMP, even though small amounts of Zelda are detectable at the histone genes via ChIP-seq. Zelda and CLAMP may have redundant activity or Zelda may have extraneous activity at the histone locus. From our results we further conclude that Zelda likely has dispensable activity at the zygotic histone locus.
Transgenic histone gene arrays lacking TAGteam sites still recruit HLB factors
Depleting zelda in the embryo has broad genome-wide consequences and results in near 100% embryonic lethality. To isolate the effect of Zelda specifically at the histone genes, without pleiotropic effects, we manipulated Zelda’s target elements in a transgenic histone gene array. The relationship between Zelda and its DNA binding motif, the TAGteam sites, is well documented (Foo et al., 2014; Liang et al., 2008; Nien et al., 2011; R. Satija & R. K. Bradley, 2012). The number of TAGteam sites within a region is directly proportional to the response to Zelda: more sites lead to more robust Zelda-dependent activation (Dufourt et al., 2018). Additionally, slight differences in the binding site sequence influence Zelda affinity and activity. Removal of TAGteam sites in certain genes directly eliminates activation of Zelda targets during early development (Li & Eisen, 2018). To eliminate the activity of Zelda specifically at the histone genes, we manipulated the TAGteam sites. It would be extremely difficult, nigh impossible, to edit the endogenous histone locus and the over 100 nearly-identical histone gene arrays (Bongartz & Schloissnig, 2019). Instead, we leveraged a transgenic system in which we can manipulate the sequence of histone array transgene outside of the larger locus to determine sequence features of the array that are required for histone gene regulation. The wild-type version of this transgene recruits all known HLB factors and expresses histone genes similar to the endogenous locus (Koreski et al., 2020; McKay et al., 2015; Rieder et al., 2017; Salzler et al., 2013).
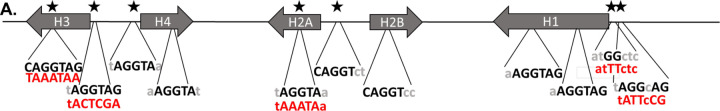
We generated a transgenic histone gene array in which we mutated 4 of the highest bound TAGteam sites to eliminate Zelda binding (Fig. 3A). We were unable to manipulate two of the TAGteam sites due to their position in the array, including a site in the histone4 promoter that overlaps with the TATA box, as these mutations could affect transcription which is necessary for full HLB formation (Salzler et al., 2013). We chose to eliminate the GG dinucleotide present in the motif because this is the most conserved and most consequential positions(Liang et al., 2008). We inserted the transgene into two genomic locations, one on Chromosome 3L (VK33) and one on Chromosome 3R (Zh86-Fb), using PhiC31 integrase, as chromatin context affects HLB formation at transgenic arrays (Salzler et al., 2013). As positive controls, we used lines carrying wild-type histone array transgenes at the same genomic sites.
Figure 3. Decreasing Zelda binding sites in transgenic histone arrays does not affect HLB factor recruitment.
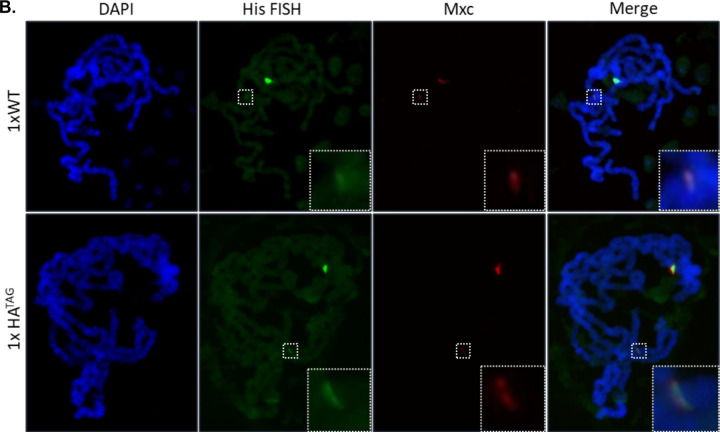
(A) We created a single histone array transgene which is not recognized by the Zelda protein (1xHATAG). Red letters represent the changes to the endogenous sequence after mutagenesis. (B) We performed polytene chromosome immunostaining on chromosomes extracted from salivary glands of flies homozygous for a single copy transgenic histone array that matches the endogenous locus sequence (1xWT) or the 1xHATAG array. We stained for the unique HLB factor Mxc and also performed DNA FISH using probes targeting histone gene sequence to identify the endogenous loci and confirm our transgenic arrays. We observe that an ectopic Mxc fluorescent band is visualizable for both the 1xWT transgene and the 1xHATAG transgene indicating that the loss of these specific Zelda binding sites does not affect HLB factor recruitment.
We tested the ability of the transgenes to recruit HLB factors by performing polytene chromosome immunostaining, as previously documented (Koreski et al., 2020; Rieder et al., 2017; Salzler et al., 2013). We visualized HLB factor recruitment through staining for the HLB-specific scaffolding protein Mxc (Kemp et al., 2021; Terzo et al., 2015). Recruitment to a single copy of the histone gene array cannot be visualized in embryos. To circumvent this issue, we use polytene chromosome immunostaining as a read out of successful HLB establishment in the embryo. Although Zelda is not expressed in larval salivary glands, the HLB is established in the early embryo, and Mxc remains associated with chromatin through cell cycles/divisions (White et al., 2011).
Our observations demonstrate that Mxc is recruited to the transgenic arrays lacking TAGteam sites (HATAG), similar to the wild-type transgenic arrays (HAWT). A single, large band near the chromocenter representing the endogenous histone locus, is visible on all polytene chromosome spreads, and serves as an internal staining control. We also observe a much smaller band representing the single array transgenes, both HAWT and HATAG, at the locations we targeted using the PhiC31 integrase (Fig. 3B). We obtained similar results when we stained for other members of the HLB, Mute and FLASH (Fig. S2). Mute is a negative regulator of histone mRNA expression (Bulchand et al., 2010), while FLASH is a core HLB component involved in transcript 3’ end processing (Duronio & Marzluff, 2017). As previously observed, the integration location of the transgene affects factor recruitment (Günesdogan, Jäckle, & Herzig, 2010; Salzler et al., 2013). We noticed a slight difference in fluorescence in the two transgenic locations: both the HAWT and HATAG transgenes have decreased fluorescence at VK33 compared to ZH86-Fb. However, we found no detectable difference in the recruitment of HLB factors to HATAG transgenes compared to HAWT transgenes at the same insertion site (Fig. S3).
The histone3/histone4 promoter sequence is targeted by both CLAMP (Rieder et al., 2017) and Zelda (Fig.1) in the early embryo. To confirm our earlier observation the zelda depletion does not impact CLAMP targeting to this region, we performed an electrophoretic mobility shit assay (EMSA). CLAMP is a member of the Late Boundary Complex, which forms during embryogenesis and is detectable by EMSA with embryo extract (Kaye et al., 2017). Probes carrying the wild-type histone3/histone4 region shift dramatically when exposed to embryo extract (Fig. S4), indicating interaction with proteins in the extract. Deleting the GA-repeats, which interact with CLAMP, from the probe abrogate this interaction. However, elimination of both TAGteam sites did not prevent the shift indicating that the embryonic interaction at this region is preserved in the absence of Zelda at the TAGteam sites. We again conclude that Zelda is likely not involved in recruiting factors to the histone genes (Fig. S4).
Zelda is likely dispensable for histone gene regulation
The 5 kb histone gene array includes at least 6 TAGteam sites that are targeted by Zelda. The TAGteam sites have slightly different sequences (Fig. 1A), which may affect Zelda binding affinity. It is possible that certain TAGteam sites are more important than others within the locus, likely exemplified by the difference in binding at each site by Zelda ChIP-seq and distribution changes across the array as development proceeds. Our transgenic histone array with mutated Zelda binding sites showed no detectable effect on HLB factor recruitment. However, it may be possible that the two TAGteam sites that remained in our array (Fig. 3A) still recruit Zelda at sufficient levels to influence histone gene regulation.
It is also possible that Zelda is important at the locus only under certain unique situations. For example, a histone array transgene lacking CLAMP-recruiting GA-repeat sequences is not targeted by HLB factors or expressed unless the endogenous histone locus is missing, indicating a “backup” or secondary mechanism of locus identification (Koreski et al., 2020; Rieder et al., 2017). While CLAMP does not target a specific region of this transgenic histone gene array by ChIP-seq, it is present in the body by immunofluorescence, suggesting that CLAMP is recruited even in the absence of DNA binding. The TAGteam sites and Zelda may be important in this unique context for CLAMP recruitment, HLB formation, and histone expression. The GA-repeats are poorly conserved in other Drosophila species, although CLAMP is still recruited to histone loci (Rieder et al., 2017; Xie et al., 2022). The TAGteam sequences may be more critical in the histone gene arrays of other Drosophila species compared to melanogaster. Therefore, Zelda may also be more critical at the locus in non-melanogaster species, either to recruit CLAMP or as an independent factor.
Overall, we conclude that although Zelda and CLAMP collaborate elsewhere in the genome during ZGA, Zelda is dispensable for proper histone gene regulation and histone locus body formation, unlike CLAMP. CLAMP remains the only known sequence-specific binding factor in Drosophila that influences histone expression and HLB formation.
Materials and Methods
Drosophila strains
We used the maternal-triple-driver (“MTD”) Gal4 stock (Bloomington, #31777) and a stock expressing shRNA against zelda from the Rushlow Lab (Sun et al., 2015) and stocks expressing shRNA against clamp (Bloomington, #57008) and mCherry (Bloomington, #35787). The Zelda-sfGFP stock was gifted from the Harrison Lab (Danielle C. Hamm et al., 2017)and was crossed to the shRNA zelda strain to generate the combined line that allowed the knock-down of the tagged protein. We maintained flies on standard cornmeal/molasses food. We maintained GAL4 crosses at 24°C and crosses/stocks for polytene chromosome preparation at 18°C.
Cloning and transgenesis
To generate the HATAG histone array transgene, we inserted a wild-type histone array sequence containing the 5 replication-dependent histone genes into a pBluescript II KS+ vector (Agilent #212207) and introduced mutations using a Q5 Site-Directed PCR Mutagenesis Kit (New England Biolabs). After mutagenesis, we transferred the array to the pMulti-BAC vector ((McKay et al., 2015)), sequence confirmed using whole plasmid sequencing (Plasmidsaurus), and inserted into the VK33 (Chr 3L) and Zh86-Fb (Chr 3R) insertion sites using φC31-mediated integration (Genetivision).
ChIP-seq data analysis and visualization
We analyzed the Zelda ChIP-seq data sets in staged early embryos by directly importing individual FASTQ data sets from Harrison et al., 2011 (NCBI GEO GSE30757) into the web-based platform Galaxy ((Afgan et al., 2016) The Galaxy Community 2023) through the NCBI SRA run selector by selecting the desired runs and utilizing the computing galaxy download feature. We retrieved the FASTQ files from the SRA using the “faster download” Galaxy command. Because the ~100 histone gene arrays are extremely similar in sequence(Bongartz & Schloissnig, 2019), we can collapse ChIP-seq data onto a single histone array (Hodkinson et al., 2023; McKay et al., 2015; Rieder et al., 2017). We used a custom “genome” that includes a single Drosophila melanogaster histone array similar to that in McKay et al. 2015, which we directly uploaded to Galaxy using the “upload data” feature and normalized using the Galaxy command “normalize fasta” specifying an 80 bp line length for the output FASTA. We aligned ChIP reads to the normalized histone gene array using Bowtie2 (Langmead & Salzberg, 2012) to create BAM files using the user built-in index and “very sensitive end-to-end” parameter settings. We converted the BAM files to bigwig files using the “bamCoverage” Galaxy command in which we set the bin size to 1 bp and set the effective genome size to user specified: 5000 bp (approximate size of l histone array). We visualized the Bigwig files using the Integrative Genome Viewer (IGV) (Robinson et al., 2011).
We performed analysis of zelda and clamp RNAi ChIP-seq data sets from Duan et al., 2021 (NCBI GEO GSE152598) as described above. We used a custom R script to combine and visualize replicates (Xie et al., 2022).
Quantitative real-time PCR
We performed qRT-PCR as described in (Urban et al., 2017) using RNA extracted from 2–4hr post-lay embryos using Trizol. We performed cDNA synthesis from RNA using LunaScript RT Supermix Kit (New England Biolabs) beginning with 500ng of RNA, which we then diluted 1:20 in MilliQ water prior to PCR. We performed reactions in technical duplicates using the AzuraQuant Green Fast qPCR Mix (Azura Genomics) and the appropriate primers. Primers for each transcript can be found in Table S1. We performed three biological replicates for each genotype and target gene. We performed PCR using the QuantStudio 3 Real-Time PCR System (ThermoFisher Scientific). We normalized transcript abundance to rp49 and calculated fold change via the ΔΔCt method (Rao et al., 2013). We analyzed data using a Student’s t-test, comparing transcript abundance between clamp or zelda RNAi embryos to matched mCherry control embryos.
Embryo immunofluorescence
We performed embryo immunofluorescence of fixed, staged embryos after aging embryos laid on grape juice plates to 2–4 hours, which we then dechorionated in 50% bleach and collected using a 40micron strainer. We immediately fixed embryos using 37% formaldehyde in heptane for 20 minutes. We then collected and washed embryos in methanol and stored in −20°C. We began immunostaining by rehydrating embryos using increasing concentrations of PB-Tween in Methanol. We then washed embryos and incubated with primary antibody in block (1% BSA in 1x PBS) overnight at 4°C on a rotator. The following day, we collected embryos and washed in block before incubating with secondary antibody for 2 hours at room temperature protected from light. We then washed and mounted embryos on slides using Prolong Diamond anti fade reagent with DAPI (ThermoFisher Scientific, P36961). We imaged embryos using a Keyence BZ-X810 Fluorescence microscope using a 20x objective. We conducted Image processing using ImageJ software (NIH).
Polytene chromosome FISH and immunofluorescence
We performed polytene chromosome FISH and immunostaining on chromosomes extracted from salivary glands dissected from third instar Drosophila larvae raised at 18°C on standard cornmeal/molasses food. We passed glands through fix 1 (4% formaldehyde, 1% Triton X-100, in 1 × PBS) for 1 min, fix 2 (4% formaldehyde, 50% glacial acetic acid) for 2 min, and 1:2:3 solution (ratio of lactic acid:water:glacial acetic acid) for 5 min prior to squashing and spreading. We washed slides in 1X PBS, then in 1% Triton X-100 (in 1X PBS) and 2 X SSC. We dehydrated slides in ethanol and allowed to air dry. We generated Biotinylated DNA FISH probes for the histone array using a Nick Translation Reaction with biotin-11-dUTP (primers found in Table S1). We then placed the slides on a heating block set to 91°C after applying the biotinylated FISH probe targeting the histone gene array in hybridization buffer (2 X SSC with dextran sulfate, formamide, and salmon sperm DNA) and sealed the coverslip with rubber cement. We incubated slides at 37°C overnight in a humid chamber. We peeled off the rubber cement and washed slides in 2 X SSC to remove coverslips and then washed in 1 X PBS. Next, we blocked for one hour in 0.5% BSA diluted in 1X PBS. We then incubated slides with primary antibodies diluted in blocking solution (antibodies specifics below) overnight at 4°C in a dark, humid chamber. We washed slides in 1 X PBS and 2 X Tween-20/NP-40 wash buffers. We next incubated the slides with streptavidin-488 (DyLight)in detection solution for 1hr in a humid chamber and then washed in 1 x PBS. We incubated slides with secondary antibody diluted in blocking solution (antibody specifics below) for two hours at room temperature. We washed and mounted slides in Prolong Diamond anti fade reagent with DAPI (ThermoFisher Scientific, P36961), and imaged chromosome spreads on a Zeiss Scope.A1 equipped with a Zeiss AxioCam using a 40×/0.75 plan neofluar objective using AxioVision software. We conducted image processing using ImageJ software (NIH).
Antibodies
We used primary antibodies at the following concentrations: guinea pig anti-Mxc (1:5000; gift from Drs. Robert Duronio and William Marzluff), rabbit anti-GFP (1:1000; ThermoFisher Scientific #A-6455), rabbit anti-FLASH (1:2000, gift from Drs. Robert Duronio and William Marzluff), guinea pig anti-Mute (1:5000; Bulchand et al. 2010). We used secondary antibodies (ThermoFisher Scientific) at a concentration of 1:1000: goat anti-guinea pig AlexFluor 647 (#A-11073), rabbit anti-guinea pig TRITC(#PA1-28594), goat anti-rabbit AlexFluor 488 (#A-21450).
EMSAs and probes
We performed EMSAs as described in (Aoki et al., 2008) with some modifications. Late embryo nuclear extracts were prepared from 6–18 hour Oregon R embryo collected on apple juice plates and aged 6 hours at room temperature. We performed nuclear extract preparations as in Aoki et al.; however, we omitted the final dialysis step and completed the extraction with the final concentration of KCl at 360 mM. We made EMSA probes using PCR using primers found in Table S1. We 5’ end labeled one pmol of probe with γ−32P-ATP (MP Biomedicals) using T4 polynucleotide kinase (NEB) in a 50μl total reaction volume at 37°C for 1 hour. We used Sephadex G-50 fine gel (Amersham Biosciences) columns to separate free ATP from labeled probes. We adjusted the volume of the eluted sample to 100 μl using deionized water so that the final concentration of the probe was 10 fmol/μl. We performed 20 μl binding reactions consisting of 0.5 μl (5 fmol) of labeled probe in the following buffer: 25 mM Tris-Cl (pH 7.4), 100 mM KCl, 1 mM EDTA, 0.1 mM dithiothreitol, 0.1 mM PMSF, 0.3 mg/ml bovine serum albumin, 10% glycerol, 0.25 mg/ml poly(dI-dC)/poly(dI-dC). We added 1 μl of nuclear extract and incubated samples at room temperature for 30 minutes. We loaded samples onto a 4% acrylamide (mono/bis, 29:1)-0.5× TBE-2.5% glycerol slab gel. We performed electrophoresis at 4°C, 180 V for 3–4 hours using 0.5× TBE-2.5% glycerol as a running buffer. We dried gels and imaged using a Typhoon 9410 scanner and Image Gauge software.
Supplementary Material
Acknowledgments
We would like to thank Drs. Robert Duronio and William Marzluff for the anti-Mcx antibody, Dr. Chris Rushlow for the anti-zelda shRNA line, and Dr. Melissa Harrison for the Zelda-sfGFP stock. We thank laboratory members for their critical reading of the manuscript.
Funding Statement
This work was supported by R35GM142724 to L.E.R and F31HD108974 to T.E.O, and T32GM00008490 to T.E.O.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References:
- Afgan E., Baker D., van den Beek M., Blankenberg D., Bouvier D., Čech M., Chilton J., Clements D., Coraor N., Eberhard C., Grüning B., Guerler A., Hillman-Jackson J., Von Kuster G., Rasche E., Soranzo N., Turaga N., Taylor J., Nekrutenko A., & Goecks J. (2016). The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res, 44(W1), W3–w10. 10.1093/nar/gkw343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo A. A., Jukam D., Straight A. F., & Skotheim J. M. (2015). Histone titration against the genome sets the DNA-to-cytoplasm threshold for the Xenopus midblastula transition. Proceedings of the National Academy of Sciences of the United States of America, 112(10), E1086–E1095. 10.1073/PNAS.1413990112/-/DCSUPPLEMENTAL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato A. (2008). DNA packaging: nucleosomes and chromatin. Nature education, 1(1), 26. [Google Scholar]
- Aoki T., Schweinsberg S., Manasson J., & Schedl P. (2008). A stage-specific factor confers Fab-7 boundary activity during early embryogenesis in Drosophila. Mol Cell Biol, 28(3), 1047–1060. 10.1128/mcb.01622-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongartz P., & Schloissnig S. (2019). Deep repeat resolution—the assembly of the Drosophila Histone Complex. Nucleic Acids Research, 47(3), e18–e18. 10.1093/NAR/GKY1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulchand S., Menon S. D., George S. E., & Chia W. (2010). Muscle wasted: A novel component of the Drosophila histone locus body required for muscle integrity. Journal of Cell Science, 123(16), 2697–2707. 10.1242/jcs.063172 [DOI] [PubMed] [Google Scholar]
- Chari S., Wilky H., Govindan J., & Amodeo A. A. (2019). Histone concentration regulates the cell cycle and transcription in early development. Development (Cambridge), 146(19). 10.1242/dev.177402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C. Y., Kemp J. P. Jr., Duronio R. J., & O’Farrell P. H. (2022). Coordinating transcription and replication to mitigate their conflicts in early Drosophila embryos. Cell Rep, 41(3), 111507. 10.1016/j.celrep.2022.111507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzis S., Elemento O., Tavazoie S., & Wieschaus E. F. (2007). Unmasking activation of the zygotic genome using chromosomal deletions in the Drosophila embryo. PLoS Biol, 5(5), e117. 10.1371/journal.pbio.0050117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J. E., Rieder L. E., Colonnetta M. M., Huang A., McKenney M., Watters S., Deshpande G., Jordan W. T., Fawzi N. L., & Larschan E. N. (2021). Clamp and zelda function together to promote drosophila zygotic genome activation. eLife, 10. 10.7554/ELIFE.69937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourt J., Trullo A., Hunter J., Fernandez C., Lazaro J., Dejean M., Morales L., Nait-Amer S., Schulz K. N., Harrison M. M., Favard C., Radulescu O., & Lagha M. (2018). Temporal control of gene expression by the pioneer factor Zelda through transient interactions in hubs. Nature Communications, 9(1). 10.1038/s41467-018-07613-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio R. J., & Marzluff W. F. (2017). Coordinating cell cycle-regulated histone gene expression through assembly and function of the Histone Locus Body. In RNA Biology (Vol. 14, pp. 726–738): Taylor and Francis Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar B. A., & Schubiger G. (1986). Parameters controlling transcriptional activation during early Drosophila development. Cell, 44(6), 871–877. 10.1016/0092-8674(86)90009-7 [DOI] [PubMed] [Google Scholar]
- Foo S. M., Sun Y., Lim B., Ziukaite R., O’Brien K., Nien C. Y., Kirov N., Shvartsman S. Y., & Rushlow C. A. (2014). Zelda potentiates morphogen activity by increasing chromatin accessibility. Curr Biol, 24(12), 1341–1346. 10.1016/j.cub.2014.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günesdogan U., Jäckle H., & Herzig A. (2010). A genetic system to assess in vivo the functions of histones and histone modifications in higher eukaryotes. EMBO Reports, 11(10), 772–776. 10.1038/embor.2010.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm D. C., & Harrison M. M. (2018). Regulatory principles governing the maternal-to-zygotic transition: Insights from Drosophila melanogaster. In Open Biology (Vol. 8): Royal Society Publishing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm D. C., Larson E. D., Nevil M., Marshall K. E., Bondra E. R., & Harrison M. M. (2017). A conserved maternal-specific repressive domain in Zelda revealed by Cas9-mediated mutagenesis in Drosophila melanogaster. PLoS Genet, 13(12), e1007120. 10.1371/journal.pgen.1007120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm D. C., Larson E. D., Nevil M., Marshall K. E., Bondra E. R., & Harrison M. M. (2017). A conserved maternal-specific repressive domain in Zelda revealed by Cas9-mediated mutagenesis in Drosophila melanogaster. PLOS Genetics, 13(12). 10.1371/journal.pgen.1007120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. M., Li X. Y., Kaplan T., Botchan M. R., & Eisen M. B. (2011). Zelda binding in the early Drosophila melanogaster embryo marks regions subsequently activated at the maternal-to-zygotic transition. PLOS Genetics, 7(10). 10.1371/journal.pgen.1002266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodkinson L. J., Smith C., Comstra H. S., Albanese E. H., Ajani B. A., Arsalan K., Daisson A. P., Forrest K. B., Fox E. H., Guerette M. R., Khan S., Koenig M. P., Lam S., Lewandowski A. S., Mahoney L. J., Manai N., Miglay J., Miller B. A., Milloway O., … Rieder L. E. (2023). A bioinformatics screen reveals Hox and chromatin remodeling factors at the Drosophila histone locus. bioRxiv. 10.1101/2023.01.06.523008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horard B., & Loppin B. (2015). Histone storage and deposition in the early Drosophila embryo. Chromosoma, 124(2), 163–175. 10.1007/s00412-014-0504-7 [DOI] [PubMed] [Google Scholar]
- Huang S. K., Whitney P. H., Dutta S., Shvartsman S. Y., & Rushlow C. A. (2021). Spatial organization of transcribing loci during early genome activation in Drosophila. Current Biology, 31(22), 5102–5110.e5105. 10.1016/J.CUB.2021.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug C. B., Grimaldi A. G., Kruse K., & Vaquerizas J. M. (2017). Chromatin Architecture Emerges during Zygotic Genome Activation Independent of Transcription. Cell, 169, 216–228. 10.1016/j.cell.2017.03.024 [DOI] [PubMed] [Google Scholar]
- Kaye E. G., Kurbidaeva A., Wolle D., Aoki T., Schedl P., & Larschan E. (2017). Drosophila Dosage Compensation Loci Associate with a Boundary-Forming Insulator Complex. Mol Cell Biol, 37(21). 10.1128/mcb.00253-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. P., Yang X. C., Dominski Z., Marzluff W. F., & Duronio R. J. (2021). Superresolution light microscopy of the Drosophila histone locus body reveals a core-shell organization associated with expression of replication-dependent histone genes. Molecular Biology of the Cell, 32(9), 942–955. 10.1091/MBC.E20-10-0645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreski K. P., Rieder L. E., McLain L. M., Chaubal A., Marzluff W. F., & Duronio R. J. (2020). Drosophila histone locus body assembly and function involves multiple interactions. Molecular Biology of the Cell, 31(14), 1525–1537. 10.1091/mbc.E20-03-0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., & Salzberg S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nature Methods, 9(4), 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larschan E., Soruco M. M., Lee O. K., Peng S., Bishop E., Chery J., Goebel K., Feng J., Park P. J., & Kuroda M. I. (2012). Identification of chromatin-associated regulators of MSL complex targeting in Drosophila dosage compensation. PLoS Genet, 8(7), e1002830. 10.1371/journal.pgen.1002830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E. D., Komori H., Fitzpatrick Z. A., Krabbenhoft S. D., Lee C.-Y., & Harrison M. (2022). Premature translation of the zygotic genome activator Zelda is not sufficient to precociously activate gene expression. bioRxiv, 2022.2003.2022.485419–482022.485403.485422.485419. 10.1101/2022.03.22.485419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-Y., & Eisen M. (2018). Effects of the maternal factor Zelda on zygotic enhancer activity in the Drosophila embryo. In: bioRxiv. [Google Scholar]
- Li Z., Thiel K., Thul P. J., Beller M., Kühnlein R. P., & Welte M. A. (2012). Lipid droplets control the maternal histone supply of Drosophila embryos. Curr Biol, 22(22), 2104–2113. 10.1016/j.cub.2012.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H. L., Nien C. Y., Liu H. Y., Metzstein M. M., Kirov N., & Rushlow C. (2008). The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature, 456(7220), 400–403. 10.1038/nature07388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. L., Murphy C., Buszczak M., Clatterbuck S., Goodman R., & Gall J. G. (2006). The Drosophila melanogaster Cajal body. J Cell Biol, 172(6), 875–884. 10.1083/jcb.200511038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff W. F., Gongidi P., Woods K. R., Jin J., & Maltais L. J. (2002). The Human and Mouse Replication-Dependent Histone Genes. Genomics, 80(5), 487–498. 10.1006/geno.2002.6850 [DOI] [PubMed] [Google Scholar]
- McCleland M. L., Shermoen A. W., & O’Farrell P. H. (2009). DNA replication times the cell cycle and contributes to the mid-blastula transition in Drosophila embryos. Journal of Cell Biology, 187(1), 7–14. 10.1083/jcb.200906191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel S. L., Gibson T. J., Schulz K. N., Fernandez Garcia M., Nevil M., Jain S. U., Lewis P. W., Zaret K. S., & Harrison M. M. (2019). Continued Activity of the Pioneer Factor Zelda Is Required to Drive Zygotic Genome Activation. Molecular Cell, 74(1), 185–195.e184. 10.1016/j.molcel.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D. J., Klusza S., Penke T. J. R., Meers M. P., Curry K. P., McDaniel S. L., Malek P. Y., Cooper S. W., Tatomer D. C., Lieb J. D., Strahl B. D., Duronio R. J., & Matera A. G. (2015). Interrogating the function of metazoan histones using engineered gene clusters. Developmental Cell, 32(3), 373–386. 10.1016/j.devcel.2014.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Q., Huang J., Chen W., Tang J., Xu C., Yu Q., Cheng Y., Ma L., Yu X., & Li S. (2017). Regulation of DNA replication-coupled histone gene expression. Oncotarget, 8(55), 95005–95022. 10.18632/ONCOTARGET.21887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. Q., Zhou R., Czech B., Liu L. P., Holderbaum L., Yang-Zhou D., Shim H. S., Tao R., Handler D., Karpowicz P., Binari R., Booker M., Brennecke J., Perkins L. A., Hannon G. J., & Perrimon N. (2011). A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nature Methods, 8(5), 405–407. 10.1038/nmeth.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nien C.-Y., Liang H.-L., Butcher S., Sun Y., Fu S., Gocha T., Kirov N., Manak J. R., & Rushlow C. (2011). Temporal Coordination of Gene Networks by Zelda in the Early Drosophila Embryo. PLOS Genetics, 7(10), e1002339–e1002339. 10.1371/journal.pgen.1002339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X., Huang X., Zhou Z., & Lin X. (2013). An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath, 3(3), 71–85. [PMC free article] [PubMed] [Google Scholar]
- Rieder L. E., Koreski K. P., Boltz K. A., Kuzu G., Urban J. A., Bowman S. K., Zeidman A., Jordan W. T., Tolstorukov M. Y., Marzluff W. F., Duronio R. J., & Larschan E. N. (2017). Histone locus regulation by the Drosophila dosage compensation adaptor protein CLAMP. Genes and Development, 31(14), 1494–1508. 10.1101/gad.300855.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. B., Sanicola M., Emmons S. W., & Childs G. (1987). Molecular characterization of the histone gene family of Caenorhabditis elegans. J Mol Biol, 196(1), 27–38. 10.1016/0022-2836(87)90508-0 [DOI] [PubMed] [Google Scholar]
- Robinson J. T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E. S., Getz G., & Mesirov J. P. (2011). Integrative genomics viewer. Nat Biotechnol, 29(1), 24–26. 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo V., & Schümperli D. (2016). Cycling in the nucleus: regulation of RNA 3’ processing and nuclear organization of replication-dependent histone genes. Current Opinion in Cell Biology, 40, 23–31. 10.1016/J.CEB.2016.01.015 [DOI] [PubMed] [Google Scholar]
- Salzler H. R., Tatomer D. C., Malek P. Y., McDaniel S. L., Orlando A. N., Marzluff W. F., & Duronio R. J. (2013). A Sequence in the Drosophila H3-H4 Promoter Triggers Histone Locus Body Assembly and Biosynthesis of Replication-Coupled Histone mRNAs. Developmental Cell, 24(6), 623–634. 10.1016/j.devcel.2013.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satija R., & Bradley R. K. (2012). The TAGteam motif facilitates binding of 21 sequence-specific transcription factors in the Drosophila embryo. Genome Research, 22(4), 656–665. 10.1101/gr.130682.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satija R., & Bradley R. K. (2012). The TAGteam motif facilitates binding of 21 sequence-specific transcription factors in the Drosophila embryo. Genome Res, 22(4), 656–665. 10.1101/gr.130682.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K. N., & Harrison M. M. (2019). Mechanisms regulating zygotic genome activation. In Nature Reviews Genetics (Vol. 20, pp. 221–234): Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal R. L., Denny P., Bruford E. A., Gribkova A. K., Landsman D., Marzluff W. F., McAndrews M., Panchenko A. R., Shaytan A. K., & Talbert P. B. (2022). A standardized nomenclature for mammalian histone genes. Epigenetics & Chromatin, 15(1), 34. 10.1186/s13072-022-00467-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Nien C. Y., Chen K., Liu H. Y., Johnston J., Zeitlinger J., & Rushlow C. (2015). Zelda overcomes the high intrinsic nucleosome barrier at enhancers during Drosophila zygotic genome activation. Genome Research, 25(11), 1703–1714. 10.1101/gr.192542.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros W., & Lipshitz H. D. (2009). The maternal-to-zygotic transition: A play in two acts. Development, 136(18), 3033–3042. 10.1242/dev.033183 [DOI] [PubMed] [Google Scholar]
- Tatomer D. C., Terzo E., Curry K. P., Salzler H., Sabath I., Zapotoczny G., McKay D. J., Dominski Z., Marzluff W. F., & Duronio R. J. (2016). Concentrating pre-mRNA processing factors in the histone locus body facilitates efficient histone mRNA biogenesis. Journal of Cell Biology, 213(5), 557–570. 10.1083/jcb.201504043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzo E. A., Lyons S. M., Poulton J. S., Temple B. R. S., Marzluff W. F., & Duronio R. J. (2015). Distinct self-interaction domains promote Multi Sex Combs accumulation in and formation of the Drosophila histone locus body. Molecular Biology of the Cell, 26(8), 1559–1574. 10.1091/mbc.E14-10-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. A., Doherty C. A., Jordan W. T. 3rd, Bliss J. E., Feng J., Soruco M. M., Rieder L. E., Tsiarli M. A., & Larschan E. N. (2017). The essential Drosophila CLAMP protein differentially regulates non-coding roX RNAs in male and females. Chromosome Res, 25(2), 101–113. 10.1007/s10577-016-9541-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. E., Burch B. D., Yang X. C., Gasdaska P. Y., Dominski Z., Marzluff W. F., & Duronio R. J. (2011). Drosophila histone locus bodies form by hierarchical recruitment of components. Journal of Cell Biology, 193(4), 677–694. 10.1083/jcb.201012077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. E., Leslie M. E., Calvi B. R., Marzluff W. F., & Duronio R. J. (2007). Developmental and cell cycle regulation of the Drosophila histone locus body. Molecular Biology of the Cell, 18(7), 2491–2502. 10.1091/mbc.E06-11-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Hodkinson L. J., Comstra H. S., Diaz-Saldana P. P., Gilbonio H. E., Gross J. L., Chavez R. M., Puckett G. L., Aoki T., Schedl P., & Rieder L. E. (2022). MSL2 targets histone genes in Drosophila virilis. bioRxiv, 2022.2012.2014.520423. 10.1101/2022.12.14.520423 [DOI] [Google Scholar]
- Yamada S., Whitney P. H., Huang S. K., Eck E. C., Garcia H. G., & Rushlow C. A. (2019). The Drosophila Pioneer Factor Zelda Modulates the Nuclear Microenvironment of a Dorsal Target Enhancer to Potentiate Transcriptional Output. Current Biology, 29(8), 1387–1393.e1385. 10.1016/j.cub.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.