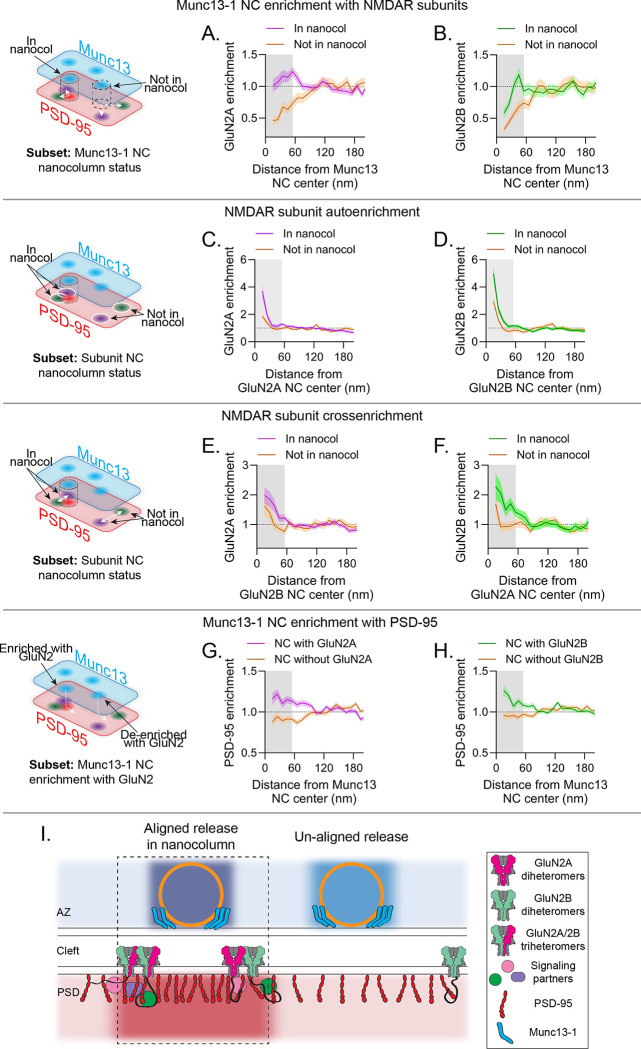
Figure 5.
Subunit-specific NMDAR nanodomains are organized with distinct trans-synaptic molecular contexts. a-h) In each row, the schematic indicates the conditional comparison being made, and each column shows the measurements made with respect to GluN2A (a,c,e,g) and GluN2B (b,d,f,h). Each panel shows the cross-enrichment plot, where data are means ± SEM shading. a-b) Munc13–1 NCs in the nanocolumn were significantly more enriched with both GluN2A and GluN2B than Munc13–1 NCs outside the nanocolumn. c-d) GluN2A and GluN2B NCs in the nanocolumn were denser than those outside the nanocolumn. e-f) GluN2A and GluN2B NCs were more cross-enriched with one another when in the nanocolumn. g-h) Munc13–1 NC enrichment with GluN2A and GluN2B can predict Munc13–1 NC enrichment with PSD-95. i) Proposed model: NMDAR distribution in synapses is governed by nanodomains with distinct trans-synaptic molecular contexts. Active zones contain molecularly diverse release sites likely with differing vesicle priming and release properties and whose functional impact depends in part on differential transsynaptic alignment. Receptor nanodomains near nanocolumn release sites contain GluN2A and GluN2B subunits; NMDARs accumulated near release sites will be activated more efficiently and potentially transduce unique signaling due to the presence of a mixed population of intracellular C-termini.