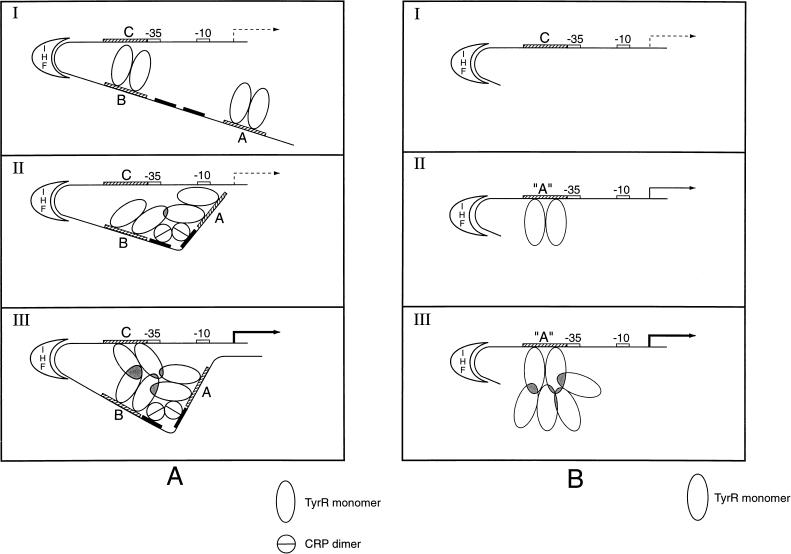
FIG. 9.
Model for involvement of IHF and CRP in TyrR-mediated activation of tpl. TyrR boxes A, B, and C are shown as hatched bars. Two CRP binding sites are shown as black bars. The ς70 promoter region is indicated by white bars. See Fig. 1 for the sequences. (A) Activation of the wild-type tpl promoter. (I) Resting state. At low tyrosine concentrations, TyrR dimers occupy strong boxes A and B, while weak box C is unoccupied. The DNA between boxes B and C is bent by IHF. (II) Catabolite repression lifted. cAMP-CRP binds to target sites, introducing a bend between boxes A and B. Interaction between TyrR dimers bound to boxes A and B, induced by DNA bending, stabilizes DNA-TyrR interaction. In states I and II, the transcriptional activity, induced by IHF, is at a very low level (indicated by a broken arrow). The mechanism of activation by IHF is not clear. (III) Tyrosine induction. At increased concentrations of tyrosine, TyrR dimers are able to occupy weak box C. Interactions between TyrR dimers bound to boxes A, B, and C become possible. The interactions between TyrR dimers are indicated by shaded regions. This gives rise to a very stable TyrR-hexamer DNA structure. This higher-order nucleoprotein complex is proposed to fully activate the tpl promoter either by inducing a conformational change within promoter DNA or by interacting directly with ς70 RNA polymerase. Activation of the tpl promoter is indicated by a heavy black arrow. (B) Model for activation of Δ179 mutant tpl promoter. A DNA segment of 179 nucleotides that included boxes A and B was deleted from the region upstream of the tpl promoter region. In a separate operation, weak box C, centered at coordinate −49.5, was replaced by a strong box essentially identical to box A (see Materials and Methods). This construct is designated box A. (I) Promoter inactivity. The Δ179 tpl promoter cannot be utilized when wild-type box C is the only TyrR target, probably because the affinity of TyrR for box C is too low to enable higher-order structures required for promoter activity to form. (II) Weak promoter activity. When box C is replaced by strong box A, TyrR dimers can attach to this region of the promoter. Occasional formation of higher-order structures would allow ς70 RNA polymerase to launch transcription. A low level of promoter activity is observed (indicated by a solid arrow). (III) Promoter activation. When the cellular content of TyrR dimers is elevated, there is an increased chance for the formation of complexes between TyrR dimers bound at box A and free TyrR dimers in solution. The formation of such higher-order complexes between TyrR dimers permits launching of ς70 RNA polymerase. Activation of the tpl promoter is indicated by a heavy black arrow.