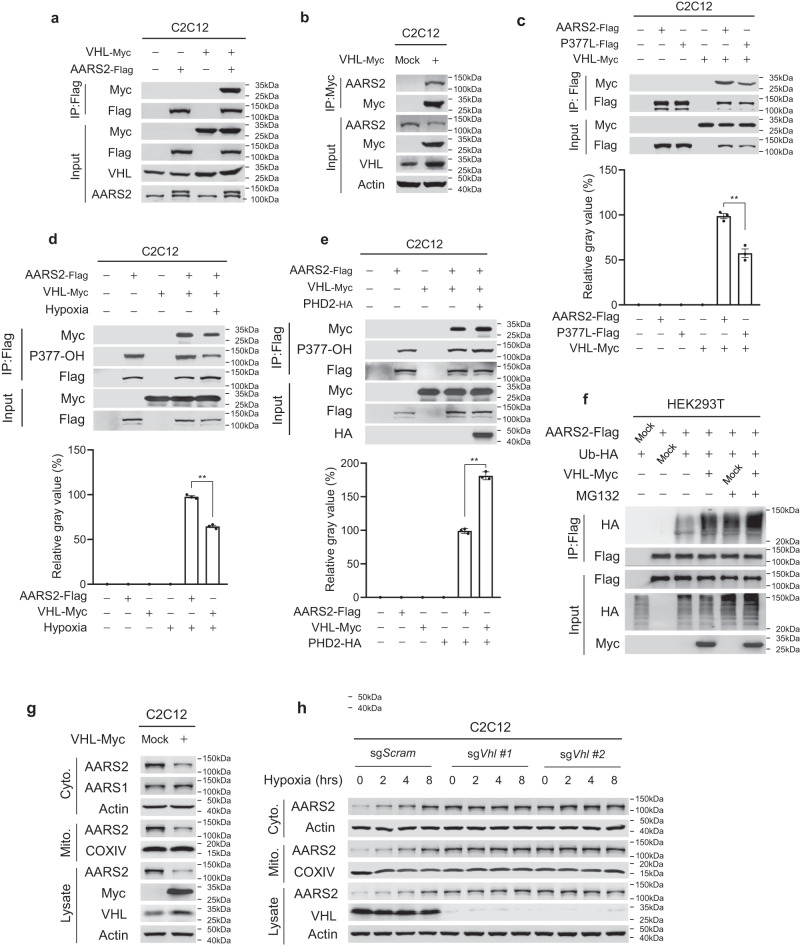
Fig. 2. AARS2 acts as a substrate of the PHD2–VHL proteasomal machinery.
a, b VHL interacts with AARS2. Co-immunoprecipitated, co-expressed (a) and endogenous (b) AARS2 was detected in C2C12 cells ectopically expressing VHL. c P377L mutant shows a weakened affinity for VHL. Interactions between VHL and AARS2, and between VHL and P377L were detected via co-immunoprecipitation when they were co-expressed in C2C12 cells. Densitometric analysis for images was provided. d, e VHL–AARS2 interaction is regulated by hypoxia and PHD2. The amount of AARS2 co-purified VHL was determined under normoxia and 8 h hypoxia (d), as well as with or without PHD2 overexpressing (e) in C2C12 cells. Densitometric analysis for images was provided. f VHL mediates proteasomal degradation. AARS2 ectopically expressed in HEK293T cells was assayed for ubiquitination when expressed alone and when co-expressed with ubiquitin or VHL, or both, under the presence and absence of MG132. g VHL downregulates AARS2 protein levels. Mitochondrial and cytosolic AARS2 levels were measured in C2C12 cells with or without VHL overexpression. h Hypoxia induces AARS2 accumulation in a VHL-dependent manner. Mitochondrial and cytosolic AARS2 levels were measured in C2C12 cells and Vhl KO C2C12 cells both cultured in a hypoxia chamber for the indicated time durations. All data are reported as mean ± SEM of three independent experiments. Statistical significance was assessed by unpaired two-tailed Student’s t-test: **P < 0.01.