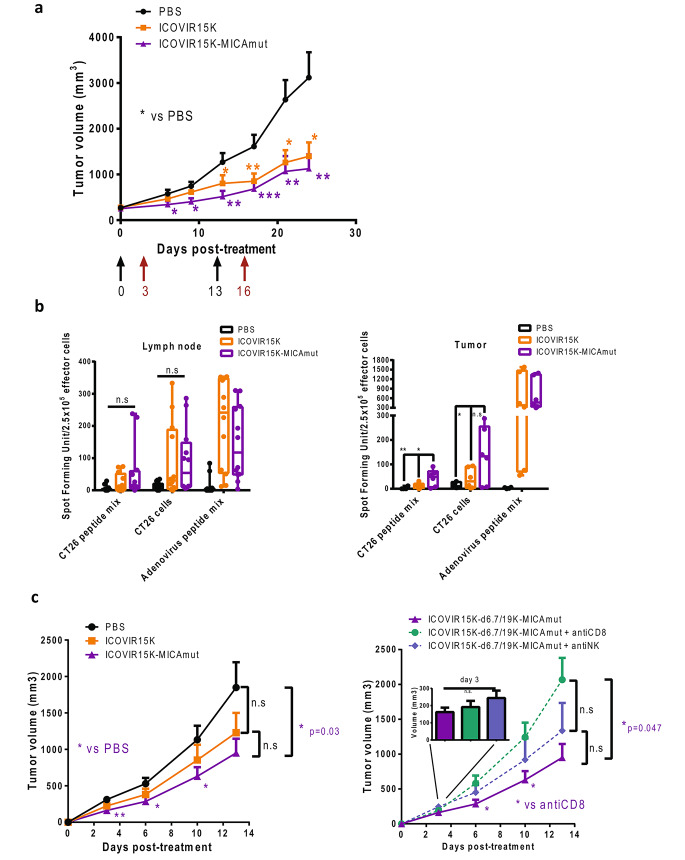
Fig. 4.
ICOVIR15K-MICAmut shows improved antitumor efficacy in vivo. (a) NSG mice bearing subcutaneous A549 tumors were intratumorally injected with PBS or ICOVIR15K or ICOVIR15K-MICAmut twice, on days 0 and 13. Three days after each virus administration, mice received an intravenous injection of 1 × 107 human PBMCs and tumor volume was periodically determined. The mean tumor volume ± SEM is shown. *p < 0.05, **p < 0.01; ***p < 0.001 ICOVIR15K-MICAmut and ICOVIR15K versus PBS group. (b) Balb/c mice bearing subcutaneous CT26-hCAR tumors were intratumorally injected with PBS, ICOVIR15K, or ICOVIR15K-MICAmut (1 × 109 TU/tumor) on days 0, 3 and 6. On day 11, mice were sacrificed and an IFN-γ ELISpot assay were performed on single-cell suspensions from lymph nodes (left) or tumors (right). Mouse lymphocytes were stimulated overnight with a mix of CT26 specific neopitopes peptides, whole CT26 cells at a ratio 1:1 or and adenovirus mix epitopes peptides in duplicates. Individual values of IFN-γ spot forming units/2.5 × 105 cells in 6 mice/group and means ± SD are plotted on the graphs. *p < 0.05, **p < 0.01 ICOVIR15K-MICAmut versus PBS and ICOVIR15K groups. (c) Left, the same animal model and treatment approach described in b) was carried out to evaluate the antitumor activity of ICOVIR15K-MICAmut. PBS injected mice were used as the control group. To monitor tumor volume, tumors were measured 2–3 times per week. The mean tumor volume ± SEM is shown. *p < 0.05, **p < 0.01 ICOVIR15K-MICAmut versus PBS group. Right, to evaluate the relevance of CD8 + T cells and NKs cells in the antitumor efficacy observed for ICOVIR15K-MICA, balb/c mice with subcutaneous CT26-hCAR tumors were injected with CD8 or NK-depleting antibodies and treated as before. Again, tumors were measured 2–3 times per week. The mean tumor volume ± SEM is shown. *p < 0.05 ICOVIR15K-MICAmut non depleted versus ICOVIR15K-MICAmut CD8-depleted group. A zoom on day 3 is shown to reflect the impact of NK cells depletion at an early time point