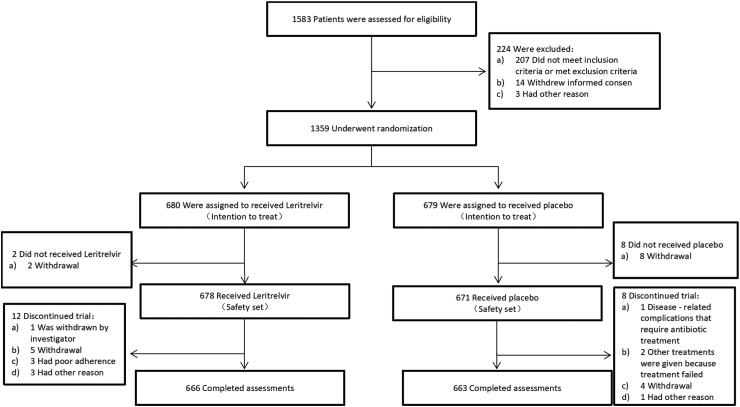
Fig. 1.
Trial profile. Eligible participants were randomly assigned in a 1:1 ratio to either the leritrelvir (RAY1216) group or the placebo group, and underwent a 5-day drug administration and 24-day follow-up. Assessments at prespecified time points included vital sign recording, physical examination, 12-lead electrocardiogram examination, clinical laboratory examination (during the screening period, day 6 ± 1, and day 29 ± 3 after the first dose), collection of nasopharyngeal swabs to quantify the SARS-CoV-2 viral load with reverse transcriptase-polymerase chain reaction (RT-PCR) assay (at day 4, 6, 10 and 15), and reporting AEs (from signing the informed consent form to the last visit or within 28 days after the first dose for patients who were withdrawn early from the trial).