Table 1.
Optimization Studiesa
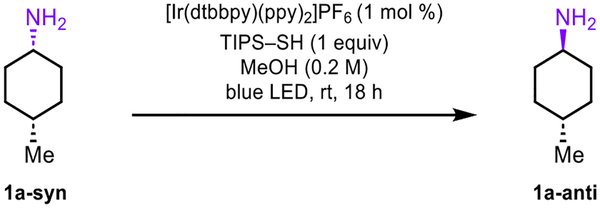
| |||
|---|---|---|---|
| entry | variation from standard conditions | Yield (%) | Dr (anti/syn) |
| 1 | none | 99 | 91:9 |
| 2 | 0.25 mol % [Ir(dtbbpy)(ppy)2]PF6 | 99 | 91:9 |
| 3 | 1-adamantanethiol | 99 | 85:15 |
| 4 | 1-butanethiol | 96 | 67:33 |
| 5 | cyclohexanethiol | 99 | 78:22 |
| 6 | thioacetic acid | 97 | 2:98 |
| 7 | TIPS-SH (0.5 equiv) | 94 | 91:9 |
| 8 | TIPS-SH (0.1 equiv) | 99 | 55:45 |
| 9 | MeCN as solvent | 89 | 90:10 |
| 10 | [Ir{dF(CF3)ppy}2(dtbpy)]PF6 | 84 | 21:79 |
Conditions: Reaction performed at 0.1 mmol of 1a-syn with the schlenk flask submitted to 3 freeze/pump/thaw cycles prior to irradiation in the photoreactor. Yields and diastereoselectivities determined by 1H NMR analysis of unpurified material with 1,3,5-trimethoxybenzene as an internal standard.
