FIGURE 2.
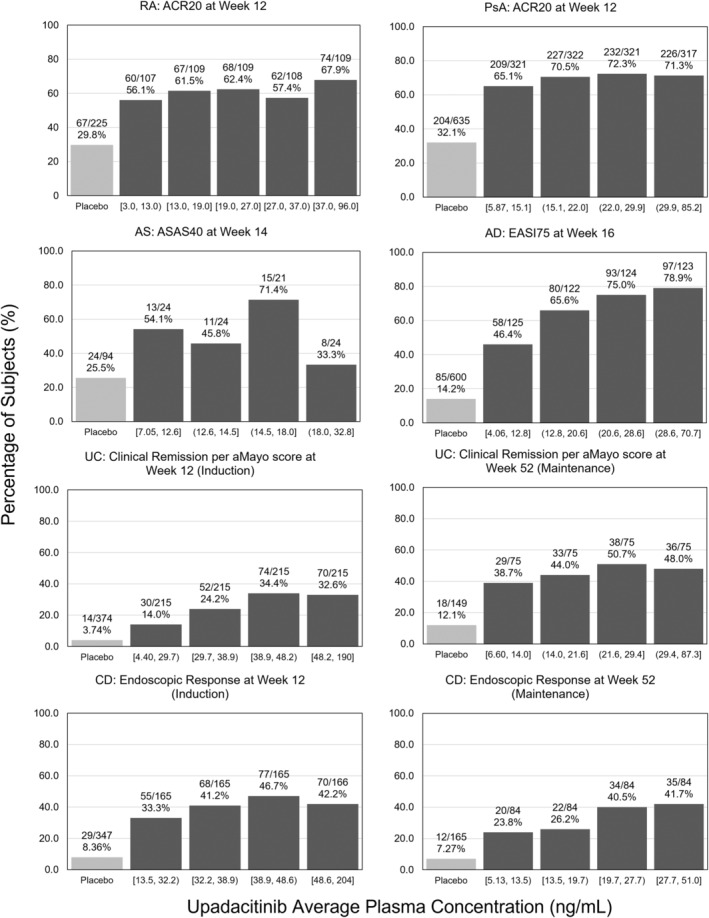
Upadacitinib exposure–response relationships for select efficacy end points by indication. ACR20, 20% improved in disease activity defined by the American College of Rheumatology; AD, atopic dermatitis; aMayo, adapted Mayo; AS, ankylosing spondylitis; ASAS40, 40% improvement from baseline defined by the Assessment in Spondyloarthritis International Society; CD, Crohn's disease; EASI75, 75% reduction from baseline in the Eczema Area and Severity Index; PsA, psoriatic arthritis; RA, rheumatoid arthritis; UC, ulcerative colitis.
