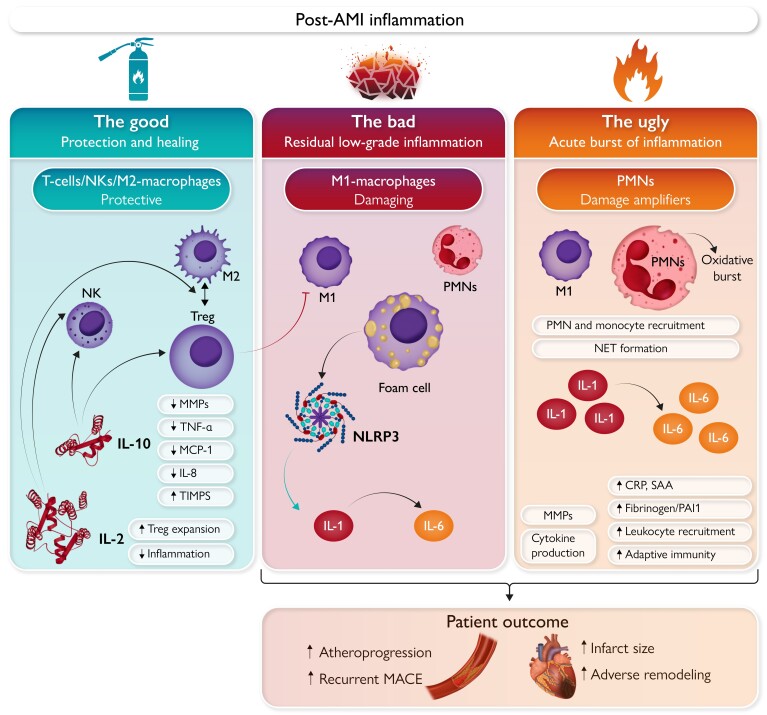
Graphical Abstract.
‘The Good’, ‘The Bad’, and ‘The Ugly’: Distinct facets of inflammation in acute myocardial infarction (AMI). The left panel (‘The Good’) shows the role of cytokines, T-cells, NKs, and macrophages in myocardial protection and healing. IL-10 and IL-2 reduce pro-inflammatory signals (e.g. TNFα, MCP-1, IL-8), extracellular matrix remodelling (MMP downregulation), while promoting Treg, Th2, and NK activation with subsequent macrophage polarization towards the M2 phenotype. The mid panel (‘The Bad’) represents the smouldering state of low-grade inflammation persisting in the late phase after AMI. Among the protagonist cellular players responsible for ‘The Bad’ are M1-polarized macrophages, foam cells, and PMNs. Induction of the NLRP3 inflammasome enhances production and secretion of IL-1α, IL-1β with subsequent enhancement of inflammatory signals via IL-6 production. These processes entertain the smouldering embers of inflammation, consequently entailing the residual inflammatory risk (RIR) that negatively affects patient outcome. The right panel focuses on ‘The Ugly’, flaming burst of excess inflammation in the early phase after AMI. PMN activation and monocytes recruitment occur upon plaque rupture and thrombosis that is further increased by NET formation. The ensuing oxidative burst contributes to damage amplification during this early phase. Cytokines which are also present in ‘The Bad’, namely IL-1 and IL-6, show a particularly excessive surge in the early phase after AMI, their damaging characteristics thus potentiated during this phase. AMI, acute myocardial infarction; CRP, C-reactive protein; IL, interleukin; MACE, major adverse cardiovascular events; MCP, monocyte chemoattractant protein; MMP, matrix metalloproteinase; NLRP, NOD-, LRR-, and pyrin domain-containing protein; NET, neutrophil extracellular trap; NK, natural killer cell; PAI1, plasminogen activator inhibitor 1; PMN, polymorphonuclear neutrophil; SAA, serum amyloid A; TIMPS, tissue inhibitors of metalloproteinases; TNF, tumour necrosis factor; Treg, regulatory T-cell.