Abstract
Purpose
Bariatric surgery is an increasingly common treatment for obesity and related comorbidities. This meta-analysis aimed to compare the outcomes of bariatric surgery and medical treatment (MT).
Materials and Methods
A systematic search of articles published from January 2013 to May 2023 identified 20 studies. The treatment arms included Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), gastric banding, and MT. The assessed outcomes included body weight loss, diabetes mellitus (DM) remission, changes in dyslipidemia and hypertension markers, and adverse events.
Results
Bariatric surgery resulted in significantly better short- and long-term weight loss than MT, with RYGB and SG showing the most substantial reduction. The DM remission rates were notably higher in the surgery group, with marked improvements in hemoglobin A1c and fasting glucose levels. Improvements in dyslipidemia were inconclusive, whereas hypertension showed modest improvements, particularly with RYGB. Complication rates varied, with RYGB reporting higher rates of early complications, and SG reporting increased rates of late complications. The perioperative reoperation rates were low across all surgical treatments. Specific adverse events, such as intestinal obstruction and anastomosis site problems, were more common in the RYGB group, whereas reflux symptoms were more common in the SG group.
Conclusion
Bariatric surgery, especially RYGB and SG, provided superior weight loss and DM remission outcomes compared to MT, although with varied complication profiles. These findings underscore the need for careful patient selection and postoperative management in bariatric surgery. Future studies should aim to refine these processes to improve patient outcomes.
Keywords: Bariatric surgery, Weight loss, Meta-analysis, Outcome assessment, Safety
INTRODUCTION
The global incidence of obesity has witnessed a concerning surge over the past 5 decades, with a mean prevalence of 19.5% in Organization for Economic Co-operation and Development (OECD) countries in 2015 [1]. This increase in obesity rates has led to a multitude of metabolic diseases, including diabetes, dyslipidemia, and fatty liver disease. Moreover, obesity significantly increases the risk of cardiovascular ailments such as hypertension, myocardial infarction, and stroke. Consequently, mortality rates have also been impacted because of the presence of obesity-related comorbidities [2,3].
Various treatment modalities have been developed to address the challenges posed by overweight and obesity. These include bariatric surgery, weight loss-inducing medications, and lifestyle modifications. Among available interventions, bariatric surgery has garnered attention as the most effective method for achieving substantial weight loss and managing diabetes [4].
To gain a comprehensive understanding of the outcomes and adverse events associated with bariatric surgery, we present an updated review focusing specifically on randomized controlled trials (RCTs) conducted from 2013 to 2023. By analyzing the latest evidence from rigorous experimental studies, this meta-analysis aimed to elucidate the efficacy and safety of bariatric surgery as a viable therapeutic approach for individuals with obesity. Our primary objective was to enhance knowledge in this evolving field and provide valuable insights for clinical decision-making.
By emphasizing the inclusion of RCTs in our meta-analysis, we aimed to strengthen the reliability and validity of our findings. A systematic synthesis of high-quality evidence will enable us to draw robust conclusions regarding the effectiveness and potential risks associated with bariatric surgery. Through this comprehensive examination, we strive to provide valuable guidance to healthcare professionals and contribute to the ongoing advancements in the field of bariatric surgery.
MATERIALS AND METHODS
1. Data sources and searches
This meta-analysis was conducted according to the latest Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [5]. A comprehensive literature search was performed using MEDLINE (via PubMed), Embase, and Scopus databases. An electronic search was conducted on May 28, 2023. The search strategy was developed across concepts of “randomized controlled trials” AND “bariatric surgery” AND (“outcomes” OR “adverse events/complications”). The search terms were adapted according to the specific requirements of each database. Results from these databases were imported into EndNote X9 (Clarivate, London, UK) for further analysis and screening.
2. Eligibility criteria
Studies were eligible only if they were RCTs or prospective cohort studies published within the time frame of January 1, 2013 to May 28, 2023. All papers needed to be written in English to be considered for review. This review considered 3 types of bariatric surgery that are commonly performed: Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and adjustable gastric banding (GB). Control group data on non-surgical treatments for obesity, such as diet, exercise, and weight-reducing drugs, were collected for comparison. For inclusion, studies had to report at least one of the following outcomes: changes in body weight, body mass index (BMI), hemoglobin A1c (HbA1c), fasting glucose level, diabetes remission rate, total cholesterol, high-density (HDL) and low-density lipoproteins (LDL), triglycerides, blood pressure, and adverse events.
3. Study selection and data collection
Two independent reviewers screened the titles and abstracts of the retrieved database files to exclude duplicate studies and studies irrelevant to the topic of research. After selecting potentially relevant studies, the reviewers read the full text to assess their eligibility. Studies lacking sufficient data on the outcomes of interest for the meta-analysis were excluded from further consideration. Additionally, pilot studies and those with a short-term follow-up duration (less than 1 year) were excluded to ensure the robustness of the analysis. Furthermore, studies focusing on overweight adolescents were excluded to reduce heterogeneity. Discrepancies or differences in opinion between the reviewers during the screening and eligibility assessment processes were resolved through discussion and consensus.
Relevant information was extracted from the eligible papers for data collection. These included details such as type of bariatric surgery performed, number of patients initially enrolled in each study, number of patients with available follow-up data, duration of the follow-up period, outcome measures of interest, and any reported adverse events. When the units of measurement varied across studies, appropriate conversions were performed to maintain consistency. In cases where standard deviations were missing, they were derived from the provided confidence intervals.
4. Statistical analysis
Meta-analysis was performed using a subset of studies obtained during the data extraction process. Statistical analyses were conducted using the STATA/MP V18 software (StataCorp LLC, College Station, TX, USA). The weighted mean changes in the outcomes of interest were calculated separately for each type of bariatric surgery or nonoperative management. To account for potential heterogeneity among the included studies, a random-effects model with the restricted maximum likelihood method was used to estimate the mean and corresponding 95% confidence interval. The degree of inconsistency across the combined study results was assessed using the I2 statistic, which quantifies the proportion of the total variation attributable to heterogeneity.
RESULTS
1. Data retrieval
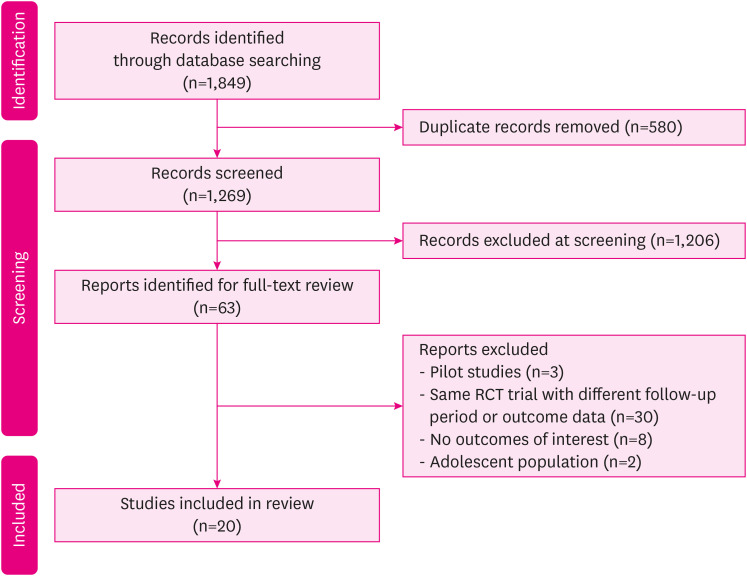
The process of searching for and selecting studies is illustrated in Fig. 1. Initially, a search was conducted in various databases for articles published from January 1, 2013 to May 28, 2023, resulting in a total of 1,849 articles. After removing duplicate records, 1,269 articles remained for screening. After careful evaluation of titles and abstracts, 1,206 articles were found to be irrelevant to our research topic and were subsequently excluded. The remaining 63 studies underwent a full-text review, leading to the exclusion of 43 articles based on the reasons provided in Fig. 1. Finally, 20 studies were deemed suitable for inclusion in the meta-analysis. Among these, there were 19 RCTs and 1 prospective cohort study.
Fig. 1. PRISMA 2020 flowchart [5].
PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled trial.
2. Study characteristics
The analysis included 20 studies with a combined 44 treatment arms. Table 1 provides a comprehensive summary of all studies included in the meta-analysis. When available, studies were referred to by trial names; otherwise, they were referenced by the first author’s name. Both short-term outcomes (1–2 years after treatment) and long-term effects (3–10 years after treatment) were analyzed. Among the treatment arms, there were 17 for RYGB surgery, 10 for SG, 5 for GB surgery, and 12 for medical treatment (MT) (Table 1). The analysis included 1,984 patients, with 843 undergoing RYGB, 540 undergoing SG, 151 undergoing GB, and 420 receiving MT.
Table 1. Summary of studies included in the meta-analysis.
| Study | Year | Country | Arms | N | Follow-up | Outcomes of interest |
|---|---|---|---|---|---|---|
| BRAVES [6] | 2023 | Italy | RYGB | 96 | 1 year | Bwt, BMI, HbA1c, fasting glucose, Lipid profiles, Complications |
| SG | 96 | |||||
| MT | 96 | |||||
| GATEWAY [7] | 2020 | Brazil | RYGB | 44 | 1 year | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| MT | 40 | 3 years | ||||
| BASE [8] | 2020 | Brazil | RYGB | 18 | 90 days | Complications |
| SG | 18 | |||||
| Horwitz et al. [9] | 2020 | United States | RYGB | 8 | 5 years | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| SG | 18 | |||||
| GB | 3 | |||||
| MT | 14 | |||||
| TRIABETES [10,11] | 2020 | United States | RYGB | 20 | 1 year | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| GB | 21 | 5 years | ||||
| MT | 20 | |||||
| MOMS [12] | 2020 | Brazil | RYGB | 51 | 2 years | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| MT | 49 | 5 years | ||||
| Simonson et al. [13] | 2019 | United States | GB | 18 | 1 year | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| MT | 22 | 3 years | ||||
| Sherf-Dagan [14] | 2019 | Israel | SG | 60 | 1 year | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| 3 years | ||||||
| Oseberg [15] | 2019 | Norway | RYGB | 54 | 1 year | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| SG | 44 | |||||
| SLIMM-T2D [16] | 2018 | United States | RYGB | 19 | 1 year | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| MT | 19 | 3 years | ||||
| SM-BOSS [17,18] | 2018 | Switzerland | RYGB | 110 | 5 years | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| SG | 107 | |||||
| Nguyen et al. [19] | 2018 | United States | RYGB | 111 | 10 years | Bwt, BMI, Remission of DM, Complications |
| GB | 86 | |||||
| SLEEVEPASS [18,20] | 2018 | Finland | RYGB | 101 | 1 year | BMI, DM, Lipid profiles, Blood pressure, Complications |
| SG | 106 | 5 years | ||||
| The Diabetes Surgery Study [21] | 2018 | United States | RYGB | 60 | 1 year | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| MT | 60 | 5 years | ||||
| STAMPEDE [22,23] | 2017 | United States | RYGB | 49 | 1 year | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| SG | 47 | 5 years | ||||
| MT | 38 | |||||
| Ignat et al. [24] | 2017 | France | RYGB | 45 | 5 years | Complications |
| SG | 55 | |||||
| Wentworth et al. [25,26] | 2017 | Austria | GB | 23 | 2 years | Bwt, BMI, DM profiles, Lipid profiles, BP |
| MT | 25 | 5 years | ||||
| CROSSROADS [27] | 2016 | United States | RYGB | 15 | 1 year | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| MT | 17 | |||||
| Mingrone et al. [28] | 2015 | Italy | RYGB | 20 | 5 years | Bwt, BMI, DM, Lipid profiles, BP, Complications |
| MT | 20 | |||||
| Keidar et al. [29] | 2013 | Israel | RYGB | 22 | 1 year | Bwt, BMI, HbA1c |
| SG | 19 |
N = number of patients enrolled, RYGB = Roux-en-Y gastric bypass surgery, SG = sleeve gastrectomy, GB = gastric banding, MT = medical treatment, Bwt = body weight change, BMI = body mass index, DM = diabetes mellitus, DM profiles = diabetes mellitus remission rates, HbA1c = fasting glucose level, Lipid profiles = total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides.
3. Outcomes
1) Body weight loss
Body weight changes were assessed as the percentage of body weight loss and percentage of BMI reduction. Some studies reported the percentage of excess weight loss (EWL); however, most of the studies provided sufficient information to calculate the percentage of total body weight loss. Therefore, %EWL analysis was not included in the meta-analysis. To account for the potential degradation of long-term effects over time, separate analyses were performed for short-term (1–2 years after the intervention) and long-term (3–10 years after the intervention) outcomes (Table 2). In short-term analysis, significant weight loss was observed in the bariatric surgery group. The weight loss percentages were as follows: −28.48% (−31.06 to −25.91) for RYGB, −25.75% (−32.51 to −19.00) for SG, −17.06% (−20.22 to −13.90) for GB, and −6.73% (−9.50 to −3.97) for MT. Remarkably, even in the long-term analysis, the weight reduction remained relatively preserved, with the following weight loss percentages observed: −25.37% (−28.88 to −21.87) for RYGB, −18.67% (−27.53 to −9.81) for SG, −12.87% (−16.51 to −9.22) for GB, and −5.10% (−9.04 to −1.16) for MT. A similar trend was observed in the analysis of changes in BMI. In the short-term, the BMI change percentages were −28.47% (−31.18 to −25.77) for RYGB, −24.94% (−32.05 to −17.83) for SG, −16.73% (−19.91 to −13.55) for GB, and −6.03% (−8.89 to −3.17) for MT. The long-term BMI change percentages were −24.96% (−28.51 to −21.41) for RYGB, −19.48% (−27.83 to −11.12) for SG, −12.58% (−16.20 to −8.96) for GB, and −4.00% (−7.67 to −0.33) for MT. Notably, the heterogeneity among the studies included in each meta-analysis was very low (I2=0.00%). These results indicate that both short- and long-term interventions resulted in significant weight loss and reduced BMI.
Table 2. Meta-analysis of weight change outcomes.
| Variables | RYGB | SG | GB | MT | ||
|---|---|---|---|---|---|---|
| ∆Bwt (%) | ||||||
| Short-term | ||||||
| Estimates (%) | −28.48 (−31.06, −25.91) | −25.75 (−32.51, −19.00) | −17.06 (−20.22, −13.90) | −6.73 (−9.50, −3.97) | ||
| No. of arms/patients | 12/640 | 7/490 | 3/62 | 10/381 | ||
| Long-term | ||||||
| Estimates (%) | −25.37 (−28.88, −21.87) | −18.67 (−27.53, −9.81) | −12.87 (−16.51, −9.22) | −5.10 (−9.04, −1.16) | ||
| No. of arms/patients | 10/560 | 5/353 | 5/149 | 8/250 | ||
| ∆BMI (%) | ||||||
| Short-term | ||||||
| Estimates (%) | −28.47 (−31.18, −25.77) | −24.94 (−32.05, −17.83) | −16.73 (−19.91, −13.55) | −6.03 (−8.89, −3.17) | ||
| No. of arms/patients | 11/612 | 7/480 | 3/62 | 9/364 | ||
| Long-term | ||||||
| Estimates (%) | −24.96 (−28.51, −21.41) | −19.48 (−27.83, −11.12) | −12.58 (−16.20, −8.96) | −4.00 (−7.67, −0.33) | ||
| No. of arms/patients | 10/536 | 5/345 | 5/149 | 9/264 | ||
Values are presented as mean (95% confidence interval).
RYGB = Roux-en-Y gastric bypass surgery, SG = sleeve gastrectomy, GB = gastric banding, MT = medical treatment; Bwt=body weight change, BMI = body mass index.
2) Diabetes remission
In the meta-analysis of laboratory data, we were unable to conduct separate analyses for short- and long-term outcomes because of the limited availability of data. Instead, we selected data from the longest follow-up period available for each study type to perform the meta-analysis. For instance, if data were available for 1 and 5 years of follow-up, we chose data from the 5-year follow-up period.
Regarding the analysis of diabetes mellitus (DM) remission rates, we observed significant rates of DM remission in the bariatric surgery group, whereas only a small number of participants in the medical therapy group achieved DM remission (Table 3). The DM remission rates were 47% (36–59%) for RYGB, 42% (29–56%) for SG, 25% (9–41%) for GB, and 5% (2–8%) for MT. Furthermore, we analyzed the changes in HbA1c (%) levels and found that individuals who underwent bariatric surgery experienced greater reductions in HbA1c levels than the control group. The changes in HbA1c levels were −0.96 (−0.56 to −1.35) for RYGB, −0.97 (−0.03 to −1.90) for SG, −0.59 (−1.24 to +0.06) for GB, and +0.08 (−0.38 to 0.54) for MT.
Table 3. Meta-analysis of comorbidity outcomes.
| Variables | RYGB | SG | GB | MT | |
|---|---|---|---|---|---|
| Diabetes remission rates | |||||
| Estimates (%) | 47 (36, 59) | 42 (29, 56) | 25 (9, 41) | 5 (2, 8) | |
| No. of arms/patients | 13/523 | 6/212 | 5/149 | 10/279 | |
| ∆ HbA1c (%) | |||||
| Estimates (%) | −0.96 (−1.35, −0.56) | −0.97 (−0.03, −1.90) | −0.59 (−1.24, +0.06) | +0.08 (−0.38, 0.54) | |
| No. of arms/patients | 14/625 | 8/472 | 4/63 | 11/327 | |
| ∆ Fasting glucose level | |||||
| Estimates (mg/DL) | −42.85 (−69.09, −16.61) | −15.67 (−40.60, +9.27) | −34.67 (−63.02, −6.31) | −2.83 (−18.39, +12.73) | |
| No. of arms/patients | 11/462 | 6/308 | 3/60 | 10/308 | |
| ∆ Total cholesterol | |||||
| Estimates (mg/DL) | −12.49 (−26.39, 1.41) | +2.88 (−32.38, 38.13) | +22.92 (−25.85, 71.69) | −17.69 (−32.49, −2.89) | |
| No. of arms/patients | 9/385 | 6/327 | 4/60 | 8/230 | |
| ∆ LDL | |||||
| Estimates (mg/DL) | −11.60 (−25.13, 1.94) | −1.36 (−32.51, 29.78) | +14.23 (−22.15, 50.61) | −17.66 (−31.79, −3.54) | |
| No. of arms/patients | 11/410 | 7/404 | 4/60 | 10/308 | |
| ∆ HDL | |||||
| Estimates (mg/DL) | +15.21 (10.99, 19.43) | +10.70 (1.77, 19.63) | +11.00 (6.24, 15.75) | +4.57 (0.63, 8.50) | |
| No. of arms/patients | 11/410 | 6/327 | 3/60 | 11/308 | |
| ∆ Triglyceride | |||||
| Estimates (mg/DL) | −71.62 (−94.20, −49.04) | −63.15 (−126.26, −0.04) | −42.73 (−67.73, −17.73) | −14.50 (−38.55, +9.56) | |
| No. of arms/patients | 11/410 | 6/327 | 4/60 | 11/308 | |
| ∆ SBP | |||||
| Estimates (mmHg) | −15.78 (−23.48, −8.09) | −2.69 (−24.32, +18.94) | +0.12 (−7.74, +7.98) | −1.34 (−9.15, +6.46) | |
| No. of arms/patients | 9/257 | 3/102 | 4/60 | 10/228 | |
| ∆ DBP | |||||
| Estimates (mmHg) | −6.18 (−10.35, −2.00) | −4.89 (−18.47, +8.69) | +1.00 (−3.10, +5.11) | −1.02 (−5.28, +3.23) | |
| No. of arms/patients | 9/257 | 3/102 | 4/60 | 10/228 | |
Values are presented as mean (95% confidence interval).
RYGB = Roux-en-Y gastric bypass surgery; SG = sleeve gastrectomy, GB = gastric banding, MT = medical treatment, HbA1c = hemoglobin A1c, LDL = low-density lipoprotein; HDL = high-density lipoprotein; SBP = systolic blood pressure; DBP = diastolic blood pressure.
A similar trend was observed for fasting glucose levels; however, owing to the small number of studies included in the SG and GB groups, the decrease in fasting glucose levels did not reach statistical significance. Specifically, the fasting glucose levels decreased by −42.85 mg/dL (−69.09 to −16.61) for RYGB, −15.67 mg/dL (−40.60 to +9.27) for SG, −34.67 mg/dL (−63.02 to −6.31) for GB, and −2.83 mg/dL (−18.39 to +12.73) for MT.
3) Dyslipidemia
A similar meta-analysis was conducted on the laboratory findings related to dyslipidemia. However, owing to limited data availability, dyslipidemia remission rates could not be included in the final analysis. Nevertheless, the analysis revealed that changes in laboratory findings related to dyslipidemia were not significantly different between the bariatric surgery and medical therapy groups, as shown in Table 3. For total cholesterol levels, the changes were: RYGB (−12.49 mg/dL, ranging from −26.39 to 1.41), SG (+2.88 mg/dL, ranging from −32.38 to 38.13), GB (+22.92 mg/dL, ranging from −25.85 to 71.69), and MT (−17.69 mg/dL, ranging from −32.49 to −2.89). Regarding LDL levels, the changes were: RYGB (−11.60 mg/dL, ranging from −25.13 to 1.94), SG (−1.36 mg/dL, ranging from −32.51 to 29.78), GB (+14.23 mg/dL, ranging from −22.15 to 50.61), and MT (−17.66 mg/dL, ranging from −31.79 to −3.54). HDL levels showed the following changes: RYGB (+15.21 mg/dL, ranging from 10.99 to 19.43), SG (+10.70 mg/dL, ranging from 1.77 to 19.63), GB (+11.00 mg/dL, ranging from 6.24 to 15.75), and MT (+4.57 mg/dL, ranging from 0.63 to 8.50). Concerning triglyceride levels, the changes were: RYGB (−71.62 mg/dL, ranging from −94.20 to −49.04), SG (−63.15 mg/dL, ranging from −126.26 to −0.04), GB (−42.73 mg/dL, ranging from −67.73 to −17.73), and MT (−14.50 mg/dL, ranging from −38.55 to +9.56). Based on these findings, the bariatric surgery group showed no significant improvements in laboratory findings related to dyslipidemia compared with the medical therapy group. The variations in these parameters were relatively modest and did not demonstrate a clear superiority of bariatric surgery over medical therapy in terms of dyslipidemia management.
4) Hypertension
The studies included in our analysis provided limited data on hypertension remission rates. Similarly, the impact of bariatric surgery on blood pressure was modest, as indicated by the analysis of lipid profiles (Table 3). Regarding systolic blood pressure, the changes observed after different types of surgeries were as follows: RYGB (−15.78 mmHg, ranging from −23.48 to −8.09), SG (−2.69 mmHg, ranging from −24.32 to +18.94), GB (+0.12 mmHg, ranging from −7.74 to +7.98), and MT (−1.34 mmHg, ranging from −9.15 to +6.46). For diastolic blood pressure (DBP), the changes recorded were: RYGB (−6.18 mmHg, ranging from −10.35 to −2.00), SG (−4.89 mmHg, ranging from −18.47 to +8.69), GB (+1.00 mmHg, ranging from −3.10 to +5.11), and MT (−1.02 mmHg, ranging from −5.28 to +3.23). These results indicate that changes in blood pressure following bariatric surgery were relatively modest across different surgical procedures. The observed reductions in systolic and DBP were most notable in the RYGB group, with smaller and less consistent changes in the SG, GB, and MT groups. However, it is important to note that the studies included in our analysis provided limited data on remission rates for hypertension. Further research is required to fully understand the impact of bariatric surgery on the management of hypertension.
4. Adverse events
The assessment of adverse events after bariatric surgery revealed significant heterogeneity in the definitions and types of complications among the included studies. This diversity extended to the timeframes used for defining complications, ranging from early events within the initial 30 days to those observed up to 1 year post-surgery. Additionally, the scope of adverse events considered varied significantly, encompassing not only physical symptoms, such as vomiting and diarrhea, but also metabolic complications, such as hypoalbuminemia and osteopenia.
1) Early complications/late complications
Early complication rates vary across different bariatric surgery types. Notably, patients who underwent RYGB experienced a higher rate of early complications (15%, 8 to 21) compared to those who underwent SG (9%, 4 to 15). However, there was insufficient evidence to draw conclusions regarding early complications in GB patients (Table 4).
Table 4. Meta-analysis of adverse events.
| Variables | RYGB | SG | GB | |
|---|---|---|---|---|
| Early complication | ||||
| Estimates (%) | 15 (8, 21) | 9 (4, 15) | N/A | |
| No. of arms/patients | 9/524 | 6/418 | 1/20 | |
| Late complication | ||||
| Estimates (%) | 35 (21, 49) | 44 (17, 72) | 57 (4, 111) | |
| No. of arms/patients | 9/611 | 5/394 | 2/106 | |
| Reoperation | ||||
| Estimates (%) | 2 (1, 4) | 1 (0, 2) | 4 (0, 7) | |
| No. of arms/patients | 9/435 | 5/297 | 2/38 | |
| Intestinal obstruction, internal hernia | ||||
| Estimates (%) | 5 (2, 7) | 1 (0, 1) | 1 (1, 2) | |
| No. of arms/patients | 13/745 | 6/418 | 3/124 | |
| Gastric stenosis | ||||
| Estimates (%) | N/A | 1 (0, 2) | N/A | |
| No. of arms/patients | 0/0 | 6/418 | 0/0 | |
| Anastomosis leak, ulcer, stenosis | ||||
| Estimates (%) | 3 (1, 4) | 1 (0, 2) | N/A | |
| No. of arms/patients | 13/745 | 6/418 | N/A | |
| Gastric band problem | ||||
| Estimates (%) | N/A | N/A | 12 (3, 22) | |
| No. of arms/patients | N/A | N/A | 3/124 | |
| Bleeding | ||||
| Estimates (%) | 1 (0, 2) | 1 (0, 2) | 2 (2, 7) | |
| No. of arms/patients | 12/634 | 6/418 | 2/38 | |
| Dumping syndrome | ||||
| Estimates (%) | 5 (2, 8) | 1 (0, 1) | N/A | |
| No. of arms/patients | 5/402 | 4/356 | 1/18 | |
| GERD | ||||
| Estimates (%) | 4 (1, 7) | 18 (5, 31) | 7 (−1, 14) | |
| No. of arms/patients | 6/463 | 5/411 | 2/104 | |
| Gallbladder stone, cholecystitis | ||||
| Estimates (%) | 3 (1, 5) | 3 (0, 5) | 4 (0, 7) | |
| No. of arms/patients | 7/322 | 3/172 | 3/124 | |
Values are presented as mean (95% confidence interval).
RYGB = Roux-en-Y gastric bypass surgery, SG = sleeve gastrectomy, GB = gastric banding, N/A = not available, GERD = gastroesophageal reflux disease.
Late complications, which included both long-term medical and surgical issues, exhibited variability among bariatric surgery types. Specifically, RYGB showed a rate of 35% (21 to 49); SG, 44% (17 to 22); and GB, 57% (4 to 111). It is important to note that owing to differences in the definitions of early and late complications, both early and late complication meta-analyses demonstrated high heterogeneity, ranging from 77.35% to 98.00%.
2) Perioperative reoperation rates
Perioperative reoperation rates, which involved additional surgical procedures performed in the immediate postoperative period, were generally low (Table 4). RYGB, SG, and GB had rates of 2% (1 to 4%), 1% (0 to 2%), and 4% (0 to 7%), respectively. Importantly, all I2 values were <40%, indicating no clinically significant heterogeneity.
3) Specific adverse events
The analysis further explored the specific adverse events associated with bariatric surgery (Table 4). Intestinal obstruction, attributed to factors such as adhesions, ileus, or internal hernias, has varying prevalence rates. RYGB patients had the highest incidence at 5% (2 to 7), followed by SG patients at 1% (0 to 1) and GB patients at 1% (1 to 2). Anastomosis site problems, including leakage, fistula, ulcer, and stenosis, were reported at rates of 3% (1 to 4) in RYGB patients and 1% (0 to 2) in SG patients. Bleeding incidents were relatively infrequent, occurring in 1% (0 to 2) of RYGB patients, 1% (0 to 2) of SG patients, and 2% (2 to 7) of GB patients. Gastric stenosis was observed in 1% (0 to 2) of SG patients. Gastric band problems, encompassing issues such as band erosion, band slippage, and malfunction, were most prevalent in GB patients, at a rate of 12% (3 to 22).
Regarding long-term complications, dumping syndrome appeared to be more frequent among RYGB patients, with a rate of 5% (2 to 8), while SG patients exhibited a rate of 1% (0 to 1). Gastroesophageal reflux disease (GERD) was most commonly reported in SG patients at 18% (5 to 31), followed by RYGB patients at 4% (1 to 7) and GB patients at 7% (−1 to 14). The rates of gallbladder stones and cholecystitis were similar across the RYGB (3%, 1 to 5), SG (3%, 0 to 5), and GB (4%, 0 to 7) groups.
4) Mortality
Only one case of mortality related to bariatric surgery was reported. In the SM-BOSS study [17], a single case of mortality was reported in association with bariatric surgery. One patient who underwent RYGB experienced a leakage at the gastrojejunostomy site. The complications progressed, resulting in multi-organ failure and ultimately leading to the patient’s death.
DISCUSSION
This intensive review and meta-analysis provided a contemporary synthesis of the efficacy of bariatric surgery by examining RCTs and prospective cohort studies published since 2013. The focus was on weight loss efficacy, metabolic improvements, and the incidence of adverse events in comparison with conventional MT. Our findings echo the benefits of bariatric surgery, revealing notable weight loss and enhanced metabolic parameters with a relatively low occurrence of surgery-related complications, reoperations, and mortality.
Substantial short- and long-term weight loss after bariatric surgery was a notable outcome in this study. Consistent with previous meta-analyses on weight reduction after bariatric surgery, our findings showed that RYGB and SG offer superior weight reduction outcomes compared to GB or MT [30,31,32]. Long-term outcomes showed that RYGB was the most effective in maintaining substantial weight loss, followed by SG, GB, and MT. The sustained reduction in both weight and BMI over an extended period suggests that bariatric surgery, particularly RYGB, is a durable solution for individuals with morbid obesity. Given the low heterogeneity across studies, these findings confirm the generalizability of the surgical benefits.
With regard to DM remission, the outcomes of bariatric surgery were significantly superior to those of medical therapy. This is consistent with existing literature, emphasizing the role of surgical intervention in the remission of type 2 diabetes, potentially reducing the long-term burden of diabetes-related complications [33,34]. The notable decrease in HbA1c and fasting glucose levels in patients who underwent bariatric surgery reinforces the hypothesis that postoperative anatomical and physiological changes contribute to improved metabolic outcomes.
However, in terms of dyslipidemia management, bariatric surgery did not show a significant advantage over medical therapy. Although triglyceride levels improved postoperatively, cholesterol management, including HDL and LDL levels, was not significantly better, reflecting the complex nature of lipid metabolism. Previous studies have reported variable results regarding the improvement of dyslipidemia after bariatric surgery. Some studies have reported that RYGB is the most effective lipid-lowering treatment, and this study showed that RYGB is generally the most effective for controlling lipid profiles [35,36]. Moreover, modest hypertension improvements were observed after RYGB and SG, consistent with previous reports on the impact of bariatric surgery on blood pressure [37]. Overall, the results of the metabolic profile improvement after bariatric surgery highlight the need for a multifaceted approach that includes lifestyle changes and pharmacotherapy, alongside weight loss strategies.
Despite these positive outcomes, the risks associated with bariatric surgery must be considered. Our analysis indicated a notable incidence of early and late complications, with significant heterogeneity among the studies. RYGB was associated with higher early complication rates compared to SG or GB, but most complications were managed with conservative care. However, the perioperative reoperation and mortality rates were low, suggesting that bariatric surgery can be safely performed.
Meanwhile, the late complication rates were higher in the SG and GB groups than in the RYGB group. Although SG and GB were safer in the perioperative period, patients tended to require medical management because of complications. A detailed exploration of specific adverse events, such as intestinal obstruction and anastomosis issues, and specific issues related to GB, such as band complications, offers valuable insights for patient counseling and risk assessment. The increased prevalence of dumping syndrome was associated with RYGB, while GERD was associated with SG, requiring attention to long-term dietary management and potential medical interventions.
It is important to acknowledge the limitations of this study. First, the characteristics of the patient groups included in this study varied significantly. Factors such as age, sex, race, and preoperative body weight differed among the RCTs included in the meta-analysis. Therefore, caution should be exercised when interpreting meta-analysis data because of variability in the subject population. Second, the number of studies available for the meta-analysis of each clinical outcome was limited, with fewer than 10 studies included in the analysis. In particular, for GB operations, only 5 RCTs investigating the effects and adverse events were available. The scarcity of RCTs for bariatric surgery contributed to the limitations of this study. Another limitation is the variation in the follow-up periods reported for each RCT. Unfortunately, not all studies provided data on both the short- and long-term outcomes of bariatric surgery, with some studies reporting only short-term outcomes and others only long-term outcomes. To address this, we compared short- and long-term body weight loss and BMI reduction for each type of surgery and MT. However, owing to the shortage of studies, we included both short- and long-term outcomes of other parameters in the meta-analysis. Additionally, other than diabetes, this study did not evaluate the effects of bariatric surgery on the remission of obesity-related diseases such as hypertension, metabolic syndrome, dyslipidemia, sleep apnea, and cardiovascular diseases. While many studies provided data on diabetes remission rates, limited data were available on the remission rates of other comorbidities. Finally, heterogeneity in outcome reporting among the included studies should be considered when interpreting the results. For instance, different studies had varying definitions of diabetes remission rates, with some even considering partial remission criteria (<7% HbA1c), whereas others focused only on complete remission using different cutoff values (6.0% vs. 6.5% HbA1c).
CONCLUSION
In conclusion, this study confirmed the substantial effects of bariatric surgery on weight loss, diabetes remission, and improvements in laboratory findings associated with metabolic syndrome and cardiovascular diseases. These findings are consistent with those of previous meta-analyses conducted using RCTs and retrospective cohort studies. Among the different types of bariatric surgeries, RYGB demonstrated the most favorable outcomes in terms of weight reduction and other measures. However, it also resulted in the highest complication rates. Conversely, GB surgeries showed the least effectiveness in weight reduction and diabetes remission, but had the lowest complication rates. When selecting a bariatric surgery for an obese patient, it is essential to carefully weigh the benefits and risks of each type of surgery to determine the most appropriate treatment option.
Footnotes
Funding: No funding was obtained for this study.
Conflict of Interest: None of the authors have any conflict of interest.
References
- 1.Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 2.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 4.Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879–887. doi: 10.1001/jama.2020.12567. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verrastro O, Panunzi S, Castagneto-Gissey L, De Gaetano A, Lembo E, Capristo E, et al. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): a multicentre, open-label, randomised trial. Lancet. 2023;401:1786–1797. doi: 10.1016/S0140-6736(23)00634-7. [DOI] [PubMed] [Google Scholar]
- 7.Schiavon CA, Bersch-Ferreira AC, Santucci EV, Oliveira JD, Torreglosa CR, Bueno PT, et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY randomized trial (Gastric Bypass to Treat Obese Patients With Steady Hypertension) Circulation. 2018;137:1132–1142. doi: 10.1161/CIRCULATIONAHA.117.032130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pajecki D, Dantas AC, Kanaji AL, de Oliveira DR, de Cleva R, Santo MA. Bariatric surgery in the elderly: a randomized prospective study comparing safety of sleeve gastrectomy and Roux-en-Y gastric bypass (BASE Trial) Surg Obes Relat Dis. 2020;16:1436–1440. doi: 10.1016/j.soard.2020.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz D, Padron C, Kelly T, Saunders JK, Ude-Welcome A, Schmidt AM, et al. Long-term outcomes comparing metabolic surgery to no surgery in patients with type 2 diabetes and body mass index 30-35. Surg Obes Relat Dis. 2020;16:503–508. doi: 10.1016/j.soard.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Courcoulas AP, Gallagher JW, Neiberg RH, Eagleton EB, DeLany JP, Lang W, et al. Bariatric surgery vs lifestyle intervention for diabetes treatment: 5-year outcomes from a randomized trial. J Clin Endocrinol Metab. 2020;105:866–876. doi: 10.1210/clinem/dgaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courcoulas AP, Goodpaster BH, Eagleton JK, Belle SH, Kalarchian MA, Lang W, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149:707–715. doi: 10.1001/jamasurg.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen RV, Pereira TV, Aboud CM, Petry TB, Lopes Correa JL, Schiavon CA, et al. Effect of gastric bypass vs best medical treatment on early-stage chronic kidney disease in patients with type 2 diabetes and obesity: a randomized clinical trial. JAMA Surg. 2020;155:e200420. doi: 10.1001/jamasurg.2020.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonson DC, Vernon A, Foster K, Halperin F, Patti ME, Goldfine AB. Adjustable gastric band surgery or medical management in patients with type 2 diabetes and obesity: three-year results of a randomized trial. Surg Obes Relat Dis. 2019;15:2052–2059. doi: 10.1016/j.soard.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Sherf-Dagan S, Zelber-Sagi S, Buch A, Bar N, Webb M, Sakran N, et al. Prospective longitudinal trends in body composition and clinical outcomes 3 years following sleeve gastrectomy. Obes Surg. 2019;29:3833–3841. doi: 10.1007/s11695-019-04057-2. [DOI] [PubMed] [Google Scholar]
- 15.Hofsø D, Fatima F, Borgeraas H, Birkeland KI, Gulseth HL, Hertel JK, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:912–924. doi: 10.1016/S2213-8587(19)30344-4. [DOI] [PubMed] [Google Scholar]
- 16.Simonson DC, Halperin F, Foster K, Vernon A, Goldfine AB. Clinical and patient-centered outcomes in obese patients with type 2 diabetes 3 years after randomization to Roux-en-Y Gastric bypass surgery versus intensive lifestyle management: the SLIMM-T2D study. Diabetes Care. 2018;41:670–679. doi: 10.2337/dc17-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterli R, Wölnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319:255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wölnerhanssen BK, Peterli R, Hurme S, Bueter M, Helmiö M, Juuti A, et al. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: 5-year outcomes of merged data from two randomized clinical trials (SLEEVEPASS and SM-BOSS) Br J Surg. 2021;108:49–57. doi: 10.1093/bjs/znaa011. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen NT, Kim E, Vu S, Phelan M. Ten-year outcomes of a prospective randomized trial of laparoscopic gastric bypass versus laparoscopic gastric banding. Ann Surg. 2018;268:106–113. doi: 10.1097/SLA.0000000000002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salminen P, Grönroos S, Helmiö M, Hurme S, Juuti A, Juusela R, et al. Effect of laparoscopic sleeve gastrectomy vs Roux-en-Y Gastric bypass on weight loss, comorbidities, and reflux at 10 years in adult patients with obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. 2022;157:656–666. doi: 10.1001/jamasurg.2022.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikramuddin S, Korner J, Lee WJ, Thomas AJ, Connett JE, Bantle JP, et al. Lifestyle intervention and medical management with vs without Roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 years in the diabetes surgery study. JAMA. 2018;319:266–278. doi: 10.1001/jama.2017.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ignat M, Vix M, Imad I, D’Urso A, Perretta S, Marescaux J, et al. Randomized trial of Roux-en-Y gastric bypass versus sleeve gastrectomy in achieving excess weight loss. Br J Surg. 2017;104:248–256. doi: 10.1002/bjs.10400. [DOI] [PubMed] [Google Scholar]
- 25.Wentworth JM, Playfair J, Laurie C, Ritchie ME, Brown WA, Burton P, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:545–552. doi: 10.1016/S2213-8587(14)70066-X. [DOI] [PubMed] [Google Scholar]
- 26.Qi QY, Playfair J, Brown WA, Burton P, O’Brien PE, Wentworth JM. Long-term impact of weight loss for people with overweight but not obesity, and with type 2 diabetes: 10-year outcomes of a randomized trial of gastric band surgery. Diabetes Obes Metab. 2023;25:1464–1472. doi: 10.1111/dom.14992. [DOI] [PubMed] [Google Scholar]
- 27.Cummings DE, Arterburn DE, Westbrook EO, Kuzma JN, Stewart SD, Chan CP, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia. 2016;59:945–953. doi: 10.1007/s00125-016-3903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 29.Keidar A, Hershkop KJ, Marko L, Schweiger C, Hecht L, Bartov N, et al. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia. 2013;56:1914–1918. doi: 10.1007/s00125-013-2965-2. [DOI] [PubMed] [Google Scholar]
- 30.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 33.Dixon JB, le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet. 2012;379:2300–2311. doi: 10.1016/S0140-6736(12)60401-2. [DOI] [PubMed] [Google Scholar]
- 34.Maggard-Gibbons M, Maglione M, Livhits M, Ewing B, Maher AR, Hu J, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 2013;309:2250–2261. doi: 10.1001/jama.2013.4851. [DOI] [PubMed] [Google Scholar]
- 35.Spivak H, Sakran N, Dicker D, Rubin M, Raz I, Shohat T, et al. Different effects of bariatric surgical procedures on dyslipidemia: a registry-based analysis. Surg Obes Relat Dis. 2017;13:1189–1194. doi: 10.1016/j.soard.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Ricci C, Gaeta M, Rausa E, Asti E, Bandera F, Bonavina L. Long-term effects of bariatric surgery on type II diabetes, hypertension and hyperlipidemia: a meta-analysis and meta-regression study with 5-year follow-up. Obes Surg. 2015;25:397–405. doi: 10.1007/s11695-014-1442-4. [DOI] [PubMed] [Google Scholar]
- 37.Wilhelm SM, Young J, Kale-Pradhan PB. Effect of bariatric surgery on hypertension: a meta-analysis. Ann Pharmacother. 2014;48:674–682. doi: 10.1177/1060028014529260. [DOI] [PubMed] [Google Scholar]