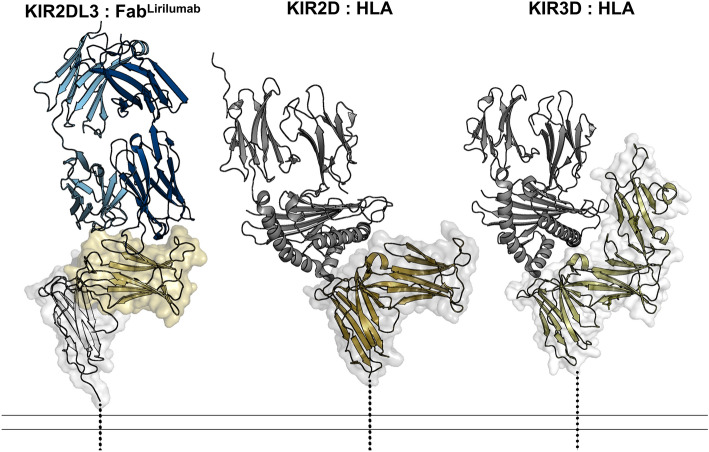
Figure 2.
The lirilumab epitope overlaps with the HLA binding site but is not exposed on KIR3D class receptors. (Left) KIR2DL3 bound to FabLirulumab, with the FabLirulumab heavy chain colored navy, light chain colored light blue, KIR2DL3 domain 1 colored gold and domain 2 colored white. (Center) KIR2DL1 bound to HLA-Cw4 (PDB 1im9)38, with HLA colored gray and KIR2DL1 colored bronze. (Right) KIR3DL1 bound to HLA-B*57:03 (PDB 6v3j)39, with HLA colored gray and KIR3DL1 colored olive. Dashed lines represent the locations of transmembrane domains. There is an approximate 134 Å2 overlap between the lirilumab epitope and the HLA binding site, revealing how lirilumab can block HLA binding. Also, these structures reveal that the lirilumab epitope is occluded by the additional domain of KIR3D receptors, explaining why lirilumab does not bind KIR3D receptors.