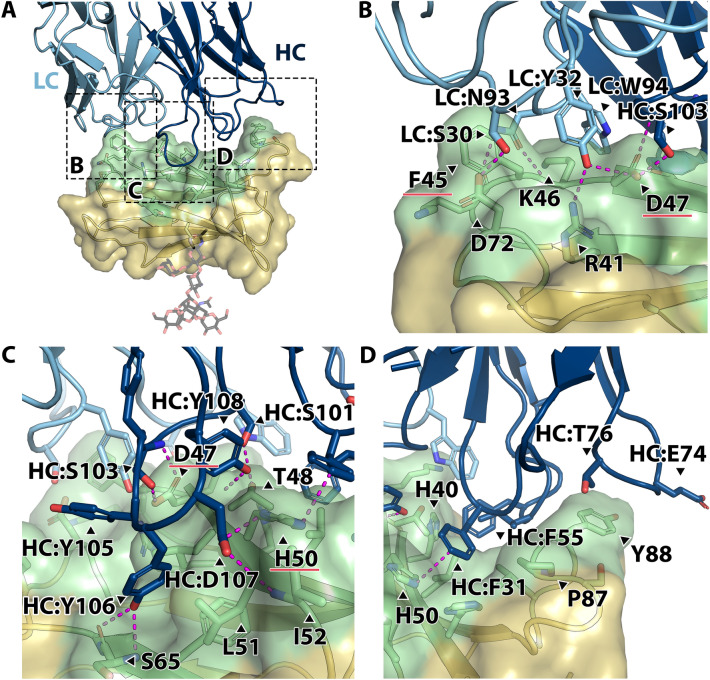
Figure 3.
Intermolecular interactions at the lirilumab:KIR2DL3 interface. (A) Interface of the FabLirilumab—KIR2DL3 complex, with the FabLirulumab heavy chain colored navy, light chain colored light blue, KIR2DL3 domain 1 colored gold, and KIR2DL3 epitope amino acids colored green. The KIR2DL3 N-glycosylation site on N63 is shown as sticks. Dashed boxes highlight the location of close-up panels (B)–(D). (B) Close-up view of lirilumab light chain interactions with KIR2DL3, with notable hydrogen bonding interactions across the interface. (C) Close-up view of lirilumab heavy chain CDR-H3 loop interactions with KIR2DL3. Note the hydrogen bonding and hydrophobic interactions by the loop residues 103–108 (SYYYDY). (D) Close-up view of lirilumab heavy chain CDR-H1 and -H2 loop interactions with KIR2DL3, which are mostly hydrophobic. In panels (B), (C), hydrogen bonds are denoted by magenta dashed lines.