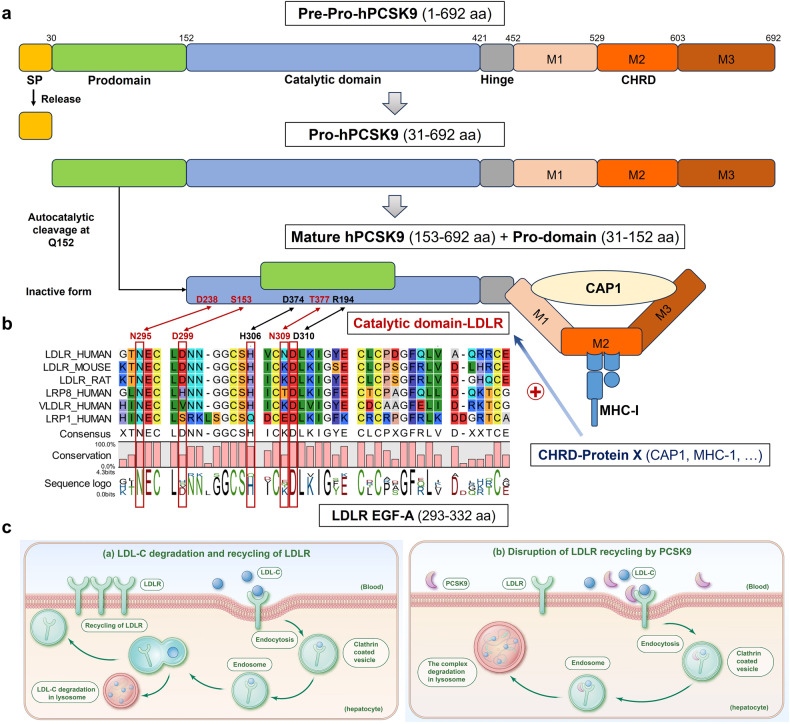
Fig. 1.
The main structure and function of PCSK9. a PCSK9 comprises a signal peptide (SP, aa 1–30), a prodomain (aa 31–152), a catalytic domain (aa 153–421) with a hinge (aa 422–452), and a Cysteine-Histidine rich C-terminal domain (CHRD, aa 453–692) that can be further divided into three modules, M1 (aa 453–529), M2 (aa 530–603), and M3 (aa 604–692). In ER, proPCSK9 undergoes autocatalytic cleavage at Q152. The prodomain is then separated from the mature PCSK9, but remains associated with the catalytic domain, inhibiting the protease activity of the mature PCSK9. b There are five residues directly involved in the avidity of PCSK9:LDLR interface including S153:D299, R194:D310, D238:N295, D374:H306, and T377:N309, in which the hydrogen bonds between D238 and N295, T377 and N309, and a salt bridge between S153 and D299 contribute to the specificity of PCSK9 binding to the epidermal growth factor (EGF)-A domain of low-density lipoprotein receptor (LDLR) instead of other EGF-like domains. Primary sequence alignments of EGF-A domain from selected species (human, mouse, and rat) and human LDLR family members (LDLR related protein 8 [LRP8]/apolipoproteinE receptor 2 [ApoER2], very low-density lipoprotein receptor [VLDLR], and LRP1) were performed using the CLC workbench. Furthermore, cyclase associated actin cytoskeleton regulatory protein 1 (CAP1) and major histocompatibility complex class 1 (MHC-1) (e.g., human leukocyte antigen [HLA]-C) may be two strong candidates for “protein X” that can promote the degradation of PCSK9-LDLR complex in acidic cytosolic compartments. c (a) LDLRs are crucial in controlling levels of LDL cholesterol (LDL-C) in the blood by managing their removal from circulation. LDLRs bind to LDL-C and the resulting complexes are internalized into hepatocytes through endocytosis into clathrin-coated vesicles that can be subsequently fused with endosomes, whose acidic environment leads to the dissociation of the LDL-C particles to be transported to lysosomes to degrade into lipids and amino acids, while LDLRs can recycle back to the surface of the hepatocytes to transport and clear additional LDL-C from the circulation. (b) When PCSK9 is secreted from hepatocytes and binds to LDLRs on the cell surface, LDLR recycling to the cell surface is impeded. Due to a conformational change in LDLR caused by PCSK9, LDLR cannot get out of the endosome to recycle back to the cell surface. Instead, the PCSK9-LDLR-LDL-C complex traffics to the lysosome for degradation. By promoting LDLR degradation, PCSK9 decreases LDLR levels at the cell surface, increasing serum LDL-C. Panels were illustrated by Adobe Illustrator and Microsoft PowerPoint