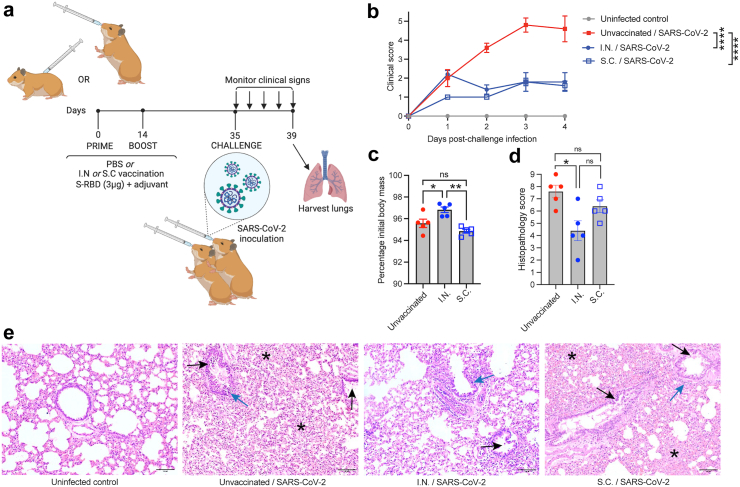
Fig. 2.
Mucosal vaccination protects hamsters from SARS-CoV-2-induced clinical disease. (a) Diagram of the experimental design where hamsters were vaccinated with S-RBD and M7 via various routes using a prime (day 0) and boost (day 14). Animals were then challenged I.N. with of 105 plaque-forming units (PFU) SARS CoV-2 on day 35 and monitored for 4 days prior to necropsy. (b) Clinical scores of vaccinated animals were significantly reduced compared to unvaccinated infected controls by 2-way ANOVA with Tukey’s post-test; p < 0.0001. n = 5 per group. (c) Day 4 post-infection, I.N. vaccinated animals began to recover body mass compared to unvaccinated and S.C. vaccinated controls that were also infected. ∗p < 0.05 and ∗∗p < 0.01 by one-way ANOVA with Tukey’s post-test. (d) I.N. vaccinated animals had reduced lung tissue damage compared to unvaccinated animals following SARS-CoV-2 challenge, by histopathological score, determined by one-way ANOVA. p < 0.05. (e) Representative images of lung histology. Scale bar = 100 μm. Black arrows indicate examples of bronchiolar epithelial cell death and desquamation, although very mild in the I.N. group. Blue arrows indicate examples peribronchiolar cellular infiltration. Asterisks are placed to indicate examples of pronounced alveolar septal infiltration.