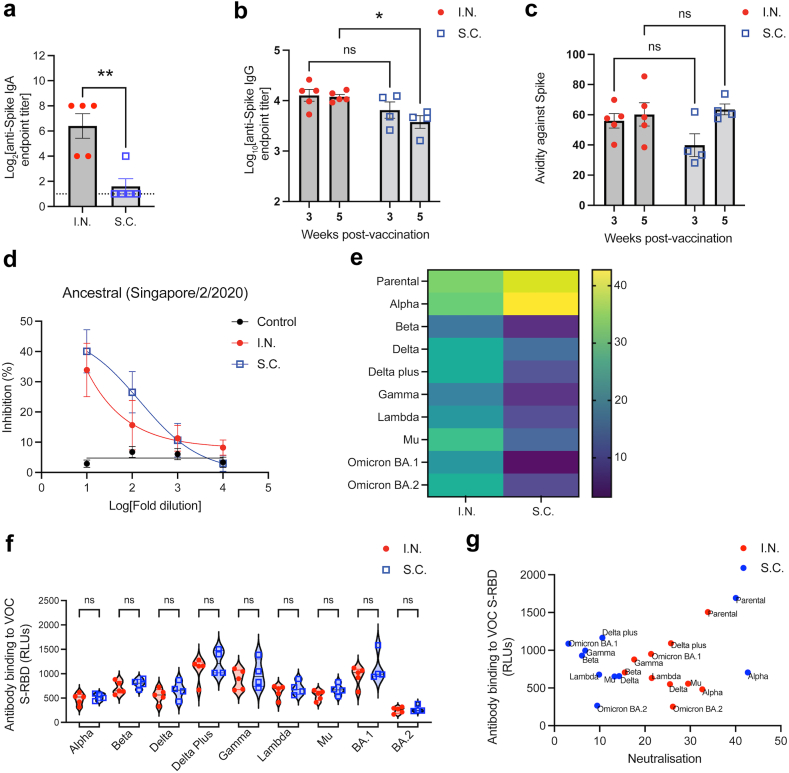
Fig. 6.
Superior antibody titre and SARS-CoV-2 variant cross-neutralisation after mucosal vaccination. (a) Anti-S-RBD IgA endpoint titres in nasal washes, 21 days post-immunisation. (b and c) Serum Anti-S-RBD IgG (b) endpoint titres and (c) avidity (percentage serum antibody that remains bound after stringent ELISA washing) following S.C. or I.N. vaccination. ∗p < 0.05, by two-way ANOVA. ns = not significant. (d) Percentage inhibition of S-RBD association with its receptor hACE-2 by serum antibodies, determined by s-VNT. For control versus I.N, p = 0.003; for control versus S.C. p < 0.001. For I.N. versus S.C., the comparison was not significantly different. (e) Heatmap depicting the % inhibition against S-RBD from multiple SARS-CoV-2 variants at 1:10 serum dilution. Corresponding dose response curves with p-values are provided in panel (d) and Supplementary Figure S5. (f) Comparison of serum antibody binding to S-RBD from multiple VOC between the I.N. and S.C. vaccination groups, determined by ELISA. RLU = relative light units. (g). No correlation between antigen binding and neutralisation (by sVNT) was observed.