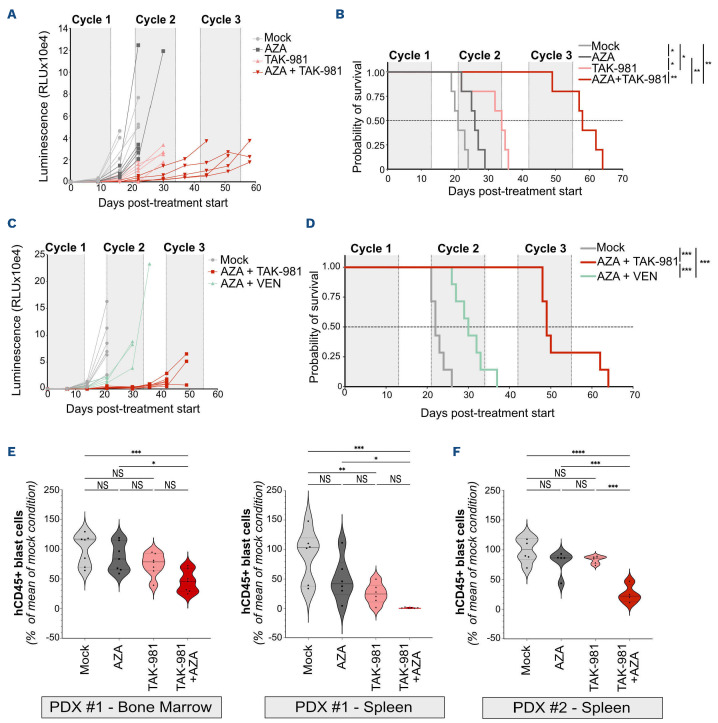
Figure 2.
TAK-981 and 5-azacytidine combination has a higher anti-leukemic activity than monotherapies in vivo. (A, B) NSG mice were injected with THP-1 cells and treated with TAK-981 (15 mg/kg, intravenously [IV]), 5-azacytidine (AZA) (2 mg/kg, intraperitoneally [IP]) or the combination according to the schedule presented in Online Supplementary Figure S2B (N=5/group). (A) Quantification, as relative luminescence units, of tumor burden evolution monitored by luminescence intensity in mice injected with bioluminescent THP-1 cells. (B) Overall survival after treatment start of mice injected with bioluminescent THP-1 cells was estimated in each group and compared using Kaplan-Meier method and log-rank test. (C, D) NSG mice were injected with THP-1 cells and treated with TAK-981 (15 mg/kg, IV) and AZA (2 mg/kg, IP) or venetoclax (VEN) (50 mg/kg, oral gavage [OG]) according to the schedule presented in Online Supplementary Figure S2D (N=7/group). (C) Quantification (as relative luminescence units) of tumor burden evolution monitored by luminescence intensity in mice injected with bioluminescent THP-1 cells. (D) Overall survival after treatment start of mice injected with bioluminescent THP-1 cells was estimated in each group of treatment and compared using Kaplan-Meier method and log-rank test. (E, F) NSG mice were injected with primary cells from 2 different AML patients. After engraftment, mice were treated with AZA and/or TAK-981 and euthanized at day 9. The total number of human CD45+ cells (hCD45) was estimated by flow cytometry in bone marrow (PDX #1, N=7) and spleen (PDX #1, N=6; PDX #2, N=4-6), and compared to the mean number of cells collected in the mock-treated group of mice. For each group, plain lines represent the median value, and dotted lines are the quartiles. Groups were compared using ordinary one-way ANOVA test.