Abstract
Background
Vertebral artery injury (VAI) is a known potential complication of posterior cervical fusion surgery. Pre-operative imaging is used to determine the patency of bilateral vertebral arteries during the planning and execution of surgery. This case illustrates an example of a staged anterior/posterior cervical reconstruction in which an iatrogenic VAI combined with a contralateral idiopathic vertebral artery dissection not identified on pre-operative imaging resulted in absent basilar artery anterograde flow.
Case Description
A 61-year-old female underwent planned staged anterior cervical decompression C4–T1 with posterior cervical fusion C2–T4 for the treatment of degenerative cervical myeloradiculopathy. During the second stage posterior fusion, iatrogenic VAI occurred during drilling for placement of the right C2 pars screw. Upon post-operative angiography, in addition to the known right VAI, there was a new left vertebral artery dissection that occurred during/after the anterior stage. The basilar artery was only filled in retrograde fashion from the right internal carotid artery across the right posterior communicating artery. The left vertebral artery dissection was treated with telescoping flow diverting stents to restore flow to the basilar artery and the right VAI was treated with coiling.
Conclusions
Surgeons should be aware of the possibility, while rare, that an occult injury to the non-injured artery is always a possibility if significant deformity correction or alignment change has occurred during cervical spine surgery. Working closely with neurointerventional colleagues can be invaluable to quickly assess and if necessary, restore blood flow to the brain through these life saving techniques.
Keywords: Vertebral artery injury (VAI), vertebral artery dissection, posterior cervical fusion, cervical deformity, case report
Highlight box.
Key findings
• We present the case of an idiopathic vertebral artery dissection after anterior cervical decompression and fusion that was discovered when treating an iatrogenic contralateral vertebral artery injury (VAI) during posterior cervical fusion.
What is known and what is new?
• VAI is a known potential complication of posterior cervical fusion surgery. This case illustrates an example of a staged anterior/posterior cervical reconstruction in which an iatrogenic VAI combined with a contralateral idiopathic vertebral artery dissection not identified on pre-operative imaging resulted in absent basilar artery anterograde flow.
What is the implication, and what should change now?
• Surgeons should be aware of the possibility, while rare, that an occult injury to the non-injured artery is always a possibility if significant deformity correction or alignment change has occurred during cervical spine surgery.
Introduction
Vertebral artery injury (VAI) is a known potential complication of both anterior and posterior cervical spine surgery. VAI can occur during soft tissue dissection, retraction, foraminotomy, drilling or instrumentation placement. The most common causes of injury have been reported to be drilling and instrumentation (1). Placement of transarticular C1–C2 screws as well as C2 pars and C2 pedicle screws carries substantial risk of injury to the vertebral artery, with some reports as high as 8.2% (2). The risk of injury is higher in patients with high riding vertebral artery (isthmus height of 5 mm or less and/or internal height of 2 mm or less on a sagittal image that is at 3 mm lateral to the cortical margin of the spinal canal wall at C2) or narrow C2 pedicles (<4 mm) (3).
Primary management options of VAI include intra-operative techniques such as tamponade/packing with a hemostatic agent, electrocoagulation, clipping/ligation or direct repair. Postoperative secondary interventional management can include coil embolization of traumatic/iatrogenic vertebrovenous fistula, stenting across a dissection or pseudoaneurysm, or, in the setting of co-dominant vertebral arteries, vessel sacrifice through combined distal and proximal occlusion.
The immediate neurologic signs and symptoms of VAI can include pseudoaneurysm, arteriovenous (AV) fistula, cerebral infarction, root damage or death. In a literature review including 69 cases, 74% had improved clinical outcome compared to the post-operative state, 13% did not improve and 8% died (5% were lost to follow-up) (1).
To mitigate the risk of iatrogenic VAI, pre-operative computed tomography (CT) or magnetic resonance imaging (MRI) angiography should be used to assess the position of the vertebral artery throughout its course in the cervical spine. Typically, the bilateral vertebral arteries originate as the first branch off the subclavian arteries and enter the transverse foramen at the C6 vertebral level in their ascent towards the foramen magnum. There is considerable redundancy of the artery between C2 and C1, which accommodates neck motion, and the artery runs in the sulcus arteriosus of the C1 posterior arch before penetrating the dura at the level of the foramen magnum. Intradurally, the vertebral arteries fuse to form the basilar artery, which provides direct arterial supply to the brainstem and bilateral thalami. Additionally, the anterior spinal artery originates from the confluence of the vertebral arteries or from the distal segment of one of the intradural vertebral arteries. There is an extensive collateral network within the cervical vasculature with vertebral artery anastomoses provided by the ascending cervical, deep cervical, occipital, and ascending pharyngeal arteries. Although most anatomic renderings of the vertebrobasilar system depict co-dominant vertebral arteries, many patients have a dominant vertebral artery with the contralateral hypoplastic vertebral artery terminating in the posterior inferior cerebellar artery.
Here we present the case of an idiopathic vertebral artery dissection in between stages of an anterior/posterior cervical decompression and fusion. In conjunction with an iatrogenic contralateral VAI during C2 pars screw placement, this resulted in no contribution to the basilar artery from either vertebral artery. We present this case in accordance with the CARE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-23-67/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The patient is a 61-year-old obese non-smoking female [body mass index (BMI): 31 kg/m2] who presented to the spine surgery clinic with 8 months of midline posterior neck pain as well as left C6, C7, and C8 radicular pain, mild hand dexterity issues and early gait dysfunction. Conservative management including acetaminophen, non-steroidal anti-inflammatory medication, gabapentin and 2 months of physical therapy was unsuccessful. She had no previous history of spine surgery. Her medical history was notable for previous alcohol and tobacco use. On examination, she had weakness in the left arm (deltoid 3/5, triceps 4/5, biceps 4/5, wrist extension 4/5) and bilateral iliopsoas weakness (4/5). She had a positive Hoffman on the left but no other pathologic reflexes. Electromyography (EMG) demonstrated left C5 and bilateral C6 radiculopathy. Pre-operative bone health assessment showed a lowest T-score of 0.3 at the femoral neck with normal vertebral fracture analysis.
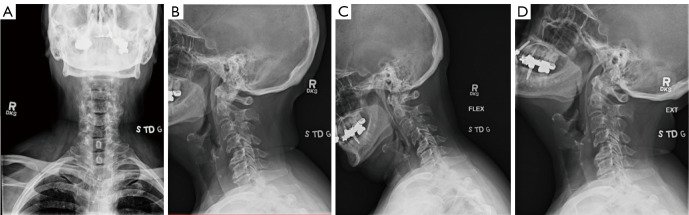
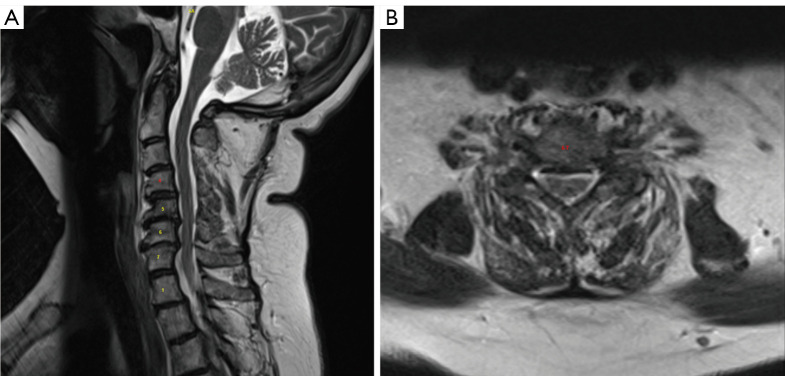
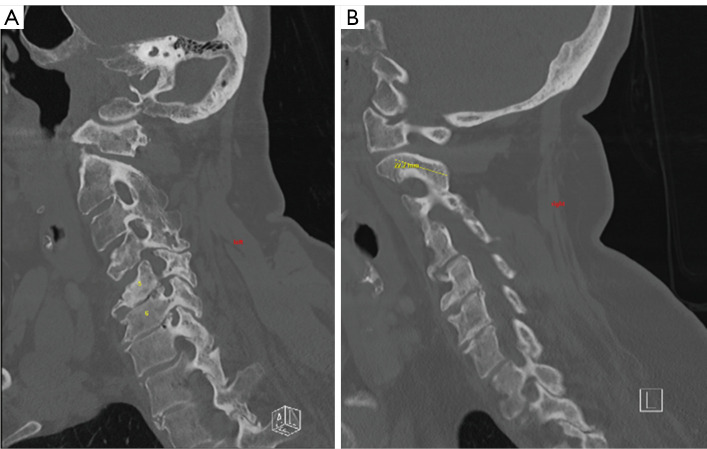
Her pre-operative imaging demonstrated a mildly kyphotic alignment of the cervical spine in the setting of multilevel spondylosis and spondylolisthesis (Figure 1). This resulted in multilevel central and foraminal stenosis most notable at C4–5, C5–6 and C6–7 (Figure 2). CT showed no high-riding vertebral artery and foraminal reconstructions showed foraminal stenosis at C4–5, C5–6, and C6–7 on the left (Figure 3).
Figure 1.
(A) AP, (B) lateral, (C) flexion and (D) extension radiographs demonstrating cervical kyphosis, spondylosis and multilevel spondylolisthesis. AP, anteroposterior.
Figure 2.
(A) Sagittal T2 MRI and (B) axial T2 MRI showing multilevel central and foraminal stenosis most notable at C4–5, C5–6 and C6–7. MRI, magnetic resonance imaging.
Figure 3.
Parasagittal CT on (A) right and (B) left showing the absence of high riding vertebral artery at C2 bilaterally. CT, computed tomography. Yellow line demonstrates planned pathway for right C2 pars screw.
Surgical options were discussed with the patient and she elected to proceed with an anterior and posterior decompression and fusion. Stage 1 of the surgery included anterior cervical diskectomy and fusion (ACDF) at C4–5 and C7–T1. A C6 corpectomy was performed along with bilateral decompressive foraminotomies at C5–6 and C6–7. An anterior cervical plate was applied C4–T1. There were no concerning intra-operative events.
The patient returned to the operating room (OR) 2 days later for scheduled posterior decompression and fusion C2–T4. The left C2 pars screw was placed without complication. During drilling of the right C2 pars screw, brisk bleeding was encountered concerning for VAI. The bleeding was controlled with bone wax in the drill hole. An entirely new C2 pars screw start point was identified, the path drilled/tapped, and screw placed without incident. The rest of the surgery continued uneventfully without any changes in neuromonitoring (motor and sensory evoked potentials and EMG). There was no hemodynamic instability. Mean arterial pressure (MAP) was kept at 85 mmHg for spinal cord perfusion, with the intra-operative MAP range 78–97 mmHg. There were no sudden rises in blood pressure that may have predisposed the patient to arterial dissection.
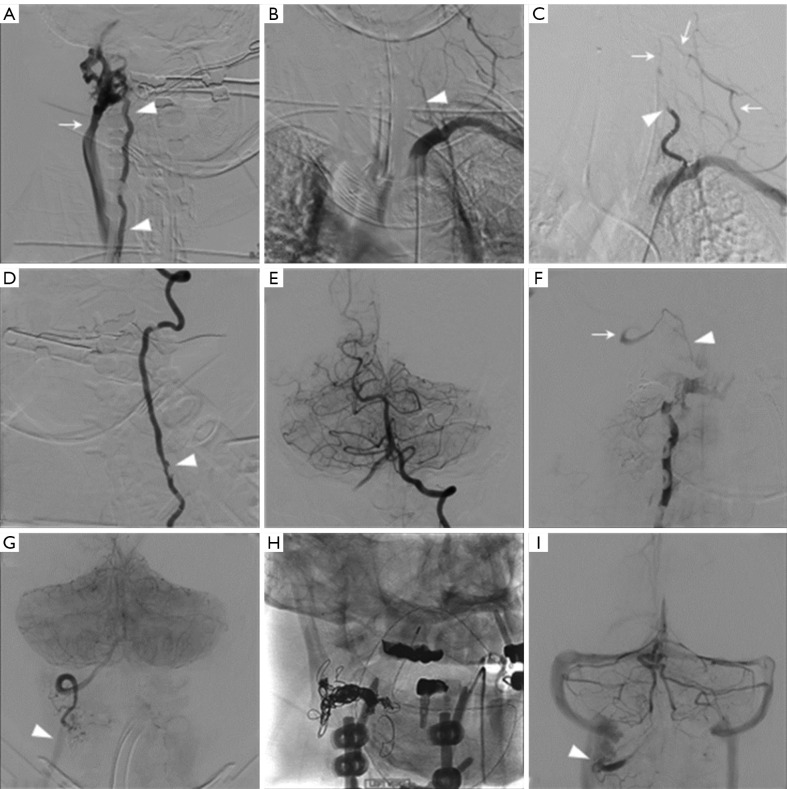
The patient was taken intubated from the OR to the neuro interventional radiology (IR) suite. While it is reasonable to have done this in the OR, the preference at our institution is to have it done in the neuro IR suite. Diagnostic angiography revealed a right vertebrovenous fistula at the site of intraoperative VAI (Figure 4A). Surprisingly, angiography of the left subclavian artery demonstrated an arterial stump corresponding to the origin of the left vertebral artery with sluggish flow that did not reach the intracranial circulation (Figure 4B). A balloon microcatheter was navigated to the site of the right vertebrovenous fistula and inflated to determine whether the vessel was in continuity beyond the arteriotomy, in which case the fistula would have been closed using coil embolization to restore normal anterograde flow. However, balloon occlusion did not reveal flow beyond the site of the fistula. Therefore, attention shifted to the left vertebral artery. Selective vertebral artery angiography revealed a focal dissection and occlusion of the midcervical vertebral artery at the C6 vertebral level with distal reconstitution by an ascending cervical artery (Figure 4C). The lesion was crossed with an Aristotle 14 microwire and Phenom 27 microcatheter and three telescoping Pipeline flow diverting stents were deployed within the cervical vertebral artery. This resulted in reopening the left vertebral artery and restoration of anterograde flow through the basilar artery (Figure 4D,4E). The patient was maintained on dual antiplatelet therapy with aspirin and ticagrelor and brought back to the angiography suite 48 hours later for elective obliteration of the right vertebrovenous fistula. A microcatheter was again navigated through the right vertebral artery to the site of fistulization and a total of 16 detachable coils were placed in the proximal draining vein of the fistula. Control angiography revealed proximal obliteration of the fistula but there was a C2–C1 arterial anastomosis that reconstituted the vertebral artery distal to the fistula within the sulcus arteriosus that displayed retrograde flow (Figure 4F). This was concerning for ongoing arteriovenous shunting from the distal vertebral artery. This was confirmed via injection of the left vertebral artery (Figure 4G). Thus, a microcatheter was navigated through the left vertebral artery into the distal right vertebral artery to the site of the fistula and placed four additional coils within the vertebral artery (Figure 4H). This resulted in distal obliteration of the fistula. Final control angiography demonstrated patency of the left vertebral artery with anterograde flow to the basilar and distal obliteration of the right vertebrovenous fistula (Figure 4I).
Figure 4.
Images of the angiography and intervention. (A) Right vertebral-venous fistula. The arrowheads point at a vertebral artery. The arrow shows external jugular. (B) Stump of the left vert (arrowhead). (C) The arrowhead shows site of vert dissection. The arrows show reconstitution of distal vert by ascending cervical. (D) This is after Pipeline placement. The arrowhead shows site of dissection that is now open. (E) Shows restored anterograde flow to the basilar from the left vert. (F) Shows proximal VVF obliteration with distal reconstitution of the V3 segment of the vert (arrow) by a small C2–C1 anastomosis (arrowhead). (G) Shows ongoing arteriovenous shunting by the distal right vertebral artery after injecting the left vert. Arrowhead shows early venous drainage. (H) Shows final coil construct at the site of the fistula. (I) Shows distal obliteration of the VVF with contrast stasis in the V3 segment of the right vert (arrowhead) and no early venous shunting. VVF, vertebrovenous fistula.
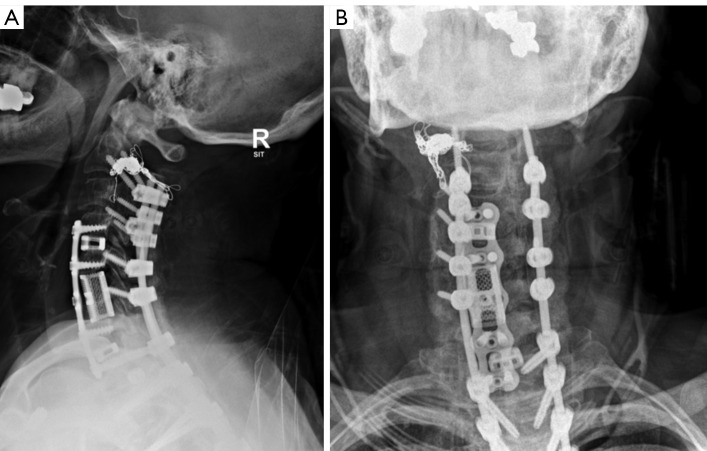
After these procedures, the patient’s neurologic exam showed decreased strength in the bilateral upper and lower extremities, left greater than right. Post-operative X-ray and CT showed appropriate positioning of implants without any complications (Figure 5). Post-operative MRI showed new cord signal abnormality C3–7 consistent with cord ischemia. The patient’s neurologic exam continued to steadily improve postoperatively but has yet to return to her neurologic baseline with residual deficits in lower greater than upper extremity strength and proprioception. At 6 weeks post-op she is ambulating with a walker with persistent lower greater than upper extremity weakness.
Figure 5.
Six-week post-operative (A) AP and (B) lateral radiographs showing cervical kyphosis correction. AP, anterioposterior.
Discussion
VAI is a known potential complication of cervical spine surgery. The immediate goals upon injury include control of local hemorrhage, prevention of immediate vertebrobasilar ischemia and prevention of cerebrovascular complications (4). Immediate treatment after injury to tamponade the bleeding includes local pressure, use of thromboplastic agents and use of bone wax. If injury is noted when drilling or tapping for screw placement, the classic teaching is that the screw can be placed to plug the hole and following the procedure the patient should be sent for angiography for potential coiling. We feel, based on the above case, that instead of placing a screw back into the bleeding hole, the surgeon should consider either redirecting for a good path of the screw or placing a short screw. We argue that using the screw to knowingly block the artery could result in absent basilar artery perfusion if the contralateral vertebral artery is not patent despite normal preoperative imaging (as evidenced in this case).
Most patients who sustain a VAI can tolerate unilateral ligation, embolization, or other obliteration of the artery. However, it poses a grave risk of neurologic compromise in those with an absent, hypoplastic or stenotic contralateral artery (4). The incidence of hypoplasia and absence of the vertebral artery are 5.7% and 1.3%, respectively, on the left and 8.8% and 3.1% on the right (5).
This case demonstrates a unique situation in which the vertebral artery was patent prior to the anterior cervical decompression and fusion but was found to be dissected upon angiography to treat the contralateral VAI. There were no intra-operative complications during the anterior stage that would have resulted in direct VAI. Rather, we believe that the change in alignment may have resulted in the dissection. In this case the alignment changed from 5 degrees kyphotic to 30 degrees lordotic, which is a substantial change. Lafage et al. reported an average cervical lordosis correction going from kyphosis (average: −11.7±18.2 degrees) to lordosis (5.5±13.4 degrees) (6). We postulate the vertebral artery may be less forgiving to changes in alignment in patients with other risk factors that predispose to vascular disease such as hypertension, high cholesterol, smoking or known coronary artery disease. The patency of the vertebral artery prior to the anterior surgery was noted on MRI. A CT angiogram may be a better study when planning a cervical deformity correction to assess the vertebral arteries preoperatively.
To our knowledge, there have been no reports of vertebral artery dissection following anterior cervical reconstruction and realignment correction. Because of the possibility of posterior circulation perfusion from the contralateral vertebral artery or other collateral flow, it is possible that this occurs without the knowledge of surgeons. It was only brought to our attention because of the angiogram performed to treat the contralateral iatrogenic VAI.
Conclusions
Here we present the case of an idiopathic vertebral artery dissection after anterior cervical decompression and fusion that was discovered when treating an iatrogenic contralateral VAI during posterior cervical fusion. Surgeons should be aware, while rare, that an occult injury to the non-injured artery is possible if significant deformity correction or alignment change has occurred throughout the procedure. This may impact what treatment decisions are made at the time of surgery and postoperatively as what occurred in our patients’ case. Working closely with your neurointerventional colleagues can be invaluable to try to quickly assess and if necessary, restore blood flow to the brain through these life saving techniques.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-23-67/rc
Peer Review File: Available at https://jss.amegroups.com/article/view/10.21037/jss-23-67/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-23-67/coif). The authors have no conflicts of interest to declare.
References
- 1.Barrie U, Detchou D, Reddy R, et al. VertebralArtery Injury with Anterior Cervical Spine Operations: A Systematic Review of Risk Factors, Clinical Outcomes, and Management Strategies. World Neurosurg 2023;173:226-236.e12. 10.1016/j.wneu.2023.02.078 [DOI] [PubMed] [Google Scholar]
- 2.Farey ID, Nadkarni S, Smith N. Modified Gallie technique versus transarticular screw fixation in C1-C2 fusion. Clin Orthop Relat Res 1999;(359):126-35. 10.1097/00003086-199902000-00013 [DOI] [PubMed] [Google Scholar]
- 3.Yeom JS, Buchowski JM, Kim HJ, et al. Risk of vertebral artery injury: comparison between C1-C2 transarticular and C2 pedicle screws. Spine J 2013;13:775-85. 10.1016/j.spinee.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 4.Bible JE, Rihn JA, Lim MR, et al. Avoiding and Managing Intraoperative Complications During Cervical Spine Surgery. J Am Acad Orthop Surg 2015;23:e81-90. 10.5435/JAAOS-D-14-00446 [DOI] [PubMed] [Google Scholar]
- 5.George B, Laurian C. The Vertebral Artery: Pathology and Surgery. New York, NY, USA: Springer-Verlag/Wien; 1987. [Google Scholar]
- 6.Lafage R, Smith JS, Fong AM, et al. Proximal and distal reciprocal changes following cervical deformity malalignment correction. J Neurosurg Spine 2022. [Epub ahead of print]. doi: . 10.3171/2022.2.SPINE211316 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as