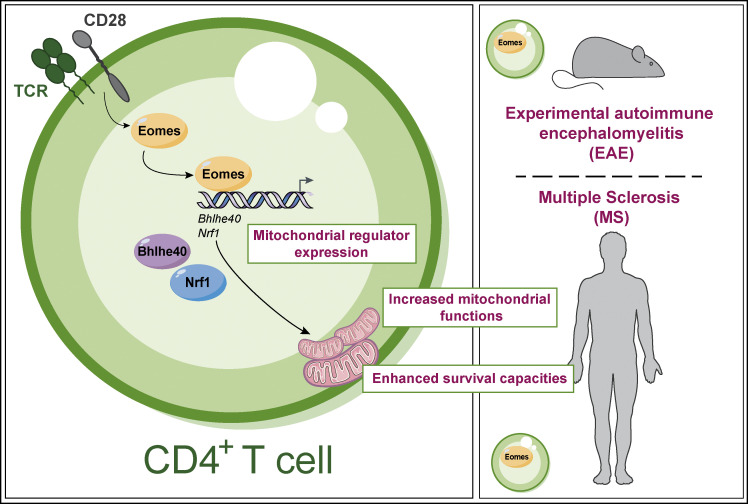
Joulia et al. reveal a new mechanism whereby the transcription factor Eomes promotes CD4+ T cell accumulation in inflamed tissue through increased mitochondrial functions and resistance to cell death, therefore promoting the severity and chronicity of inflammation.
Abstract
The mechanisms whereby Eomes controls tissue accumulation of T cells and strengthens inflammation remain ill-defined. Here, we show that Eomes deletion in antigen-specific CD4+ T cells is sufficient to protect against central nervous system (CNS) inflammation. While Eomes is dispensable for the initial priming of CD4+ T cells, it is required for long-term maintenance of CNS-infiltrating CD4+ T cells. We reveal that the impact of Eomes on effector CD4+ T cell longevity is associated with sustained expression of multiple genes involved in mitochondrial organization and functions. Accordingly, epigenetic studies demonstrate that Eomes supports mitochondrial function by direct binding to either metabolism-associated genes or mitochondrial transcriptional modulators. Besides, the significance of these findings was confirmed in CD4+ T cells from healthy donors and multiple sclerosis patients. Together, our data reveal a new mechanism by which Eomes promotes severity and chronicity of inflammation via the enhancement of CD4+ T cell mitochondrial functions and resistance to stress-induced cell death.
Graphical Abstract
Introduction
The transcription factor (TF) Eomesodermin (Eomes) is a key regulator of the development and function of immune cells. Its function has mainly been studied in natural killer (NK) and CD8+ T cells. Eomes is mandatory for NK cell development and maturation (Daussy et al., 2014; Gordon et al., 2012) and proper differentiation of cytotoxic CD8+ T cells via regulation of Ifng, Grzb, and Prf1 gene expression (Cruz-Guilloty et al., 2009; Pearce et al., 2003). It is also crucial for memory CD8+ T cell homeostasis and survival (Banerjee et al., 2010; Intlekofer et al., 2005), likely by inducing expression of the Il2rb gene that encodes the common IL-2/IL-15 receptor β chain. In addition, Eomes upregulation during chronic viral infections was associated with CD8+ T cell exhaustion (Paley et al., 2012), while its downregulation promotes type 17 response (Intlekofer et al., 2008) and tissue-resident memory (Trm) CD8+ T cell development (Mackay et al., 2015).
Nevertheless, the precise role of Eomes in the CD4+ T cell compartment remains elusive and depends on the pathological context and on T helper (Th) subsets (Dejean et al., 2019). In Th1 cells, Eomes promotes IFN-γ production (Steiner et al., 2011; Suto et al., 2006; Yang et al., 2008), cytotoxicity (Curran et al., 2013; Qui et al., 2011; Raveney et al., 2015), and GM-CSF secretion (Stienne et al., 2016). In Th2 cells, Eomes inhibits IL-5 production (Endo et al., 2011) while it promotes Th9 cytotoxicity (Lu et al., 2018) and IL-10 secretion in Tr1 (Gruarin et al., 2019; Zhang et al., 2017). Eomes also represses Th17 cell features (Ichiyama et al., 2011), and its deletion favors the accumulation of Foxp3+ regulatory CD4+ T cells in aged mice (Lupar et al., 2015). Altogether, these studies show that Eomes is expressed in nearly all Th subsets in both mice and humans. This suggests that rather than defining a CD4+ T cell lineage, Eomes expression is a hallmark of a T cell state that is not yet well understood.
The importance of Eomes in pathological inflammation was first highlighted in genetic studies showing that Eomes is a susceptibility gene for several immune-mediated diseases including multiple sclerosis (MS), rheumatoid arthritis (RA; Laufer et al., 2019; International Multiple Sclerosis Genetics Consortium, 2019), and cancer (Law et al., 2017). Eomes expression in CD4+ T cells has been associated with pathogenic Th cell populations in neuroinflammation (Raveney et al., 2015, 2021; Stienne et al., 2016; Zhang et al., 2019) and RA (Chemin et al., 2018). Conversely, other studies have shown that Eomes plays regulatory roles, dampening allergic airway inflammation and graft-versus-host disease severity (Zhang et al., 2017). A similar discrepancy was reported in cancer models, wherein Eomes+ CD4+ T cells were either required for anti-tumor immune response and associated with good prognosis or, inversely, correlated with a state of T cell exhaustion and tumor relapse (Roessner et al., 2021; Wang et al., 2012).
While the function of Eomes in the pathophysiology of these diseases is still debated, it has been consistently reported that Eomes+ CD4+ T cells accumulate in the inflamed tissues of patients with chronic inflammatory disorders. Eomes+ CD4+ T cells are strongly enriched in the inflamed gut of inflammatory bowel disease (IBD) patients (Gruarin et al., 2019) and in skin lesions from psoriasis patients (Šahmatova et al., 2017). Such accumulation has also been reported in cerebrospinal fluid from patients in a progressive state of MS (Raveney et al., 2015, 2021) and the synovial fluid of RA (Chemin et al., 2018) or juvenile idiopathic arthritis (JIA) patients (Mazzoni et al., 2019). Additional studies have described increased proportions of Eomes+ cells within tumor-infiltrating CD4+ T cell populations in both humans and mice (Pirozyan et al., 2020). However, the mechanisms by which Eomes favors tissue accumulation of CD4+ T cells in these diseases remain poorly understood.
Here, we use the experimental autoimmune encephalomyelitis (EAE) model of neuroinflammation to uncover the molecular mechanisms by which Eomes favors CD4+ T cell accumulation in inflamed tissue and strengthens chronic inflammation. Our study demonstrates that Eomes is specifically required for the persistence of central nervous system (CNS)–infiltrating CD4+ T cells by coordinating pathways involved in mitochondrial adaptation and survival capacity of pathogenic effector CD4+ T cells in inflamed tissues.
Results
Eomes is necessary for the pathogenic functions of myelin oligodendrocyte glycoprotein MOG–specific CD4+ T cells
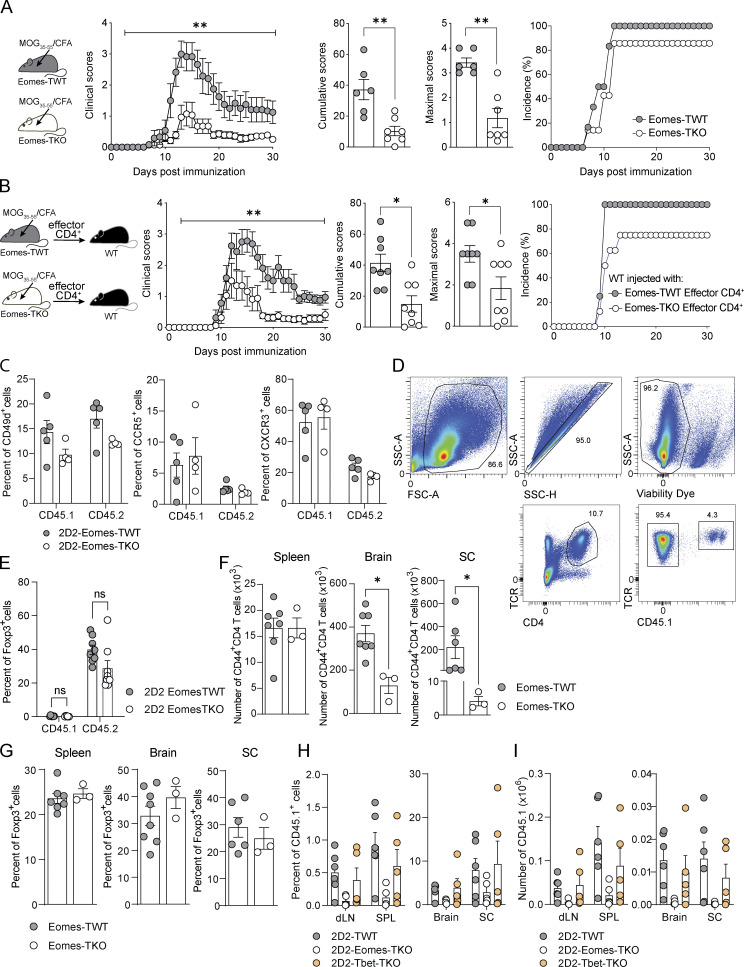
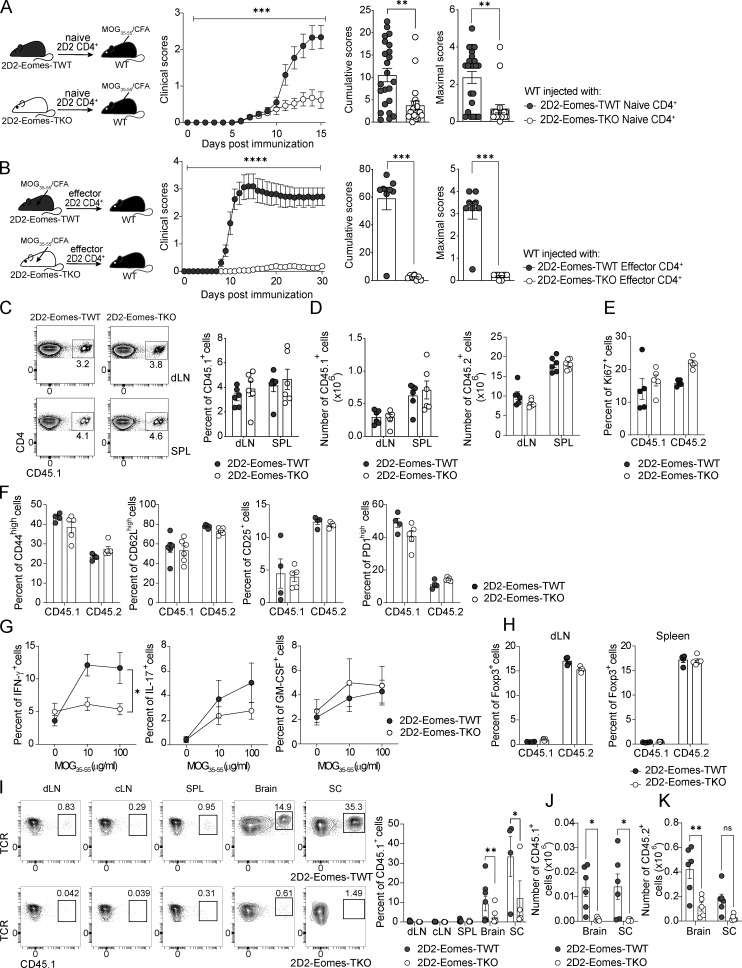
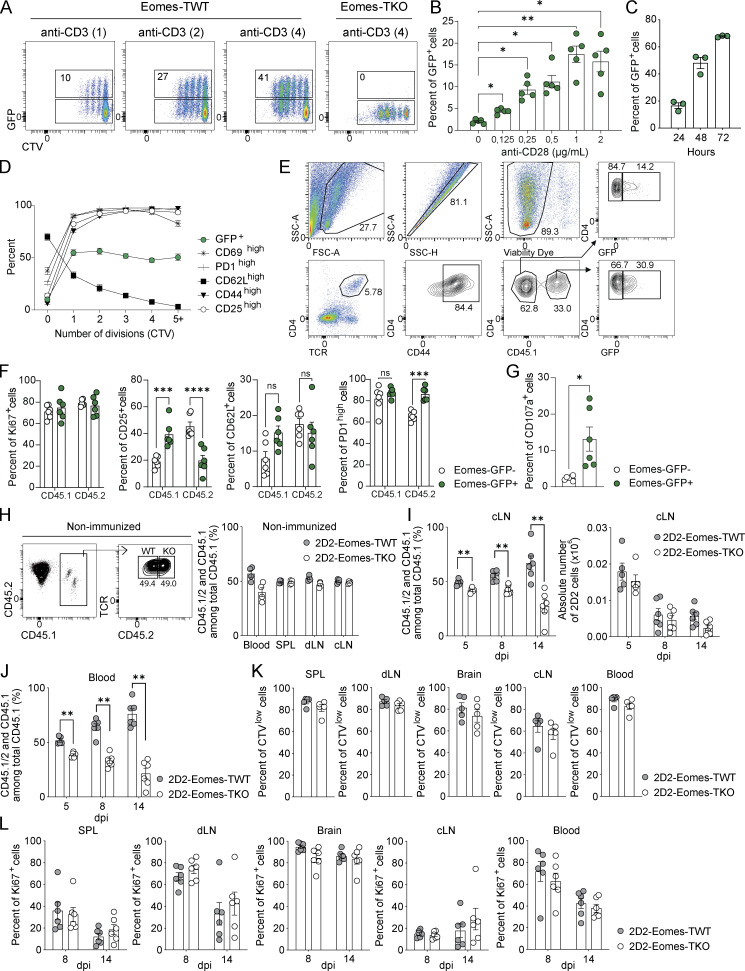
To study the role of Eomes in neuroinflammation, we immunized mice carrying a conditional deletion of Eomes in T cells (Eomesfl/fl -CD4Cre+, hereafter called Eomes-TKO) and control littermates (Eomesfl/fl-CD4Cre−, hereafter referred as Eomes-TWT) with myelin oligodendrocyte glycoprotein (MOG35–55) peptide emulsified in complete Freund’s adjuvant (CFA) along with pertussis toxin. We showed that the absence of Eomes in both CD4+ and CD8+ T cells strikingly reduced EAE severity (Fig. S1 A). These results are in agreement with previous studies, although it was reported that the absence/reduction of Eomes expression showed a more modest phenotype that could be due to either the genetic background of the mice used or the sanitary status of animal facilities. To address the role of Eomes in antigen-specific CD4+ T cells, we crossed MOG35–55-specific TCR transgenic mice, referred to as 2D2 mice (Bettelli et al., 2003), with Eomes-TKO and Eomes-TWT mice to produce 2D2-Eomes-TKO and 2D2-Eomes-TWT mice, respectively. WT mice were injected with naïve (CD62Lhigh, CD44low, and CD25−) CD4+ T cells purified from either 2D2-Eomes-TKO or 2D2-Eomes-TWT mice expressing the congenic marker CD45.1. Both sets of CD45.2 recipient mice were then immunized with MOG35–55 and CFA. 2D2-Eomes-TKO induced less severe EAE than control 2D2-Eomes-TWT (Fig. 1 A). We also observed decreased EAE severity using passive EAE models induced upon injection of 2D2 (Fig. 1 B) or polyclonal (Fig. S1 B) CD4+ T cells deficient for Eomes as compared with Eomes WT cells. Altogether, these results demonstrate that Eomes is crucial for the acquisition of pathogenic functions by MOG-specific CD4+ T cells.
Figure S1.
Eomes deletion reduces EAE severity without impacting migratory capacities or Treg compartment. (A) Eomes-TWT (n = 6) and Eomes-TKO mice (n = 7) were immunized with MOG35–55 peptide emulsified in CFA and injected i.v. with pertussis toxin. Clinical scores were evaluated daily and cumulative and maximal scores were calculated. Incidence is shown. (B) Eomes-TWT (n = 8) and Eomes-TKO (n = 8) mice were immunized with MOG35–55 peptide emulsified in CFA. At 10 dpi, cells from dLN and spleen were collected and stimulated with MOG35–55 in the presence of anti-IFN-γ mAb and IL-23. CD4+ T cells were then purified and injected i.v. into WT C57BL/6J recipients (n = 8 mice per group) and clinical scores were evaluated and cumulative and maximal scores were calculated. Incidence is shown. (C) Expression of migratory markers was analyzed in CD45.1+ and CD45.2+ compartments from mice injected with either 2D2-Eomes-TWT or 2D2-Eomes-TKO cells at 8 dpi in the spleen (n = 5 and 4 mice/group). (D) Representative gating strategy showing CD45.1+ and CD45.2+ CD4+ T cell compartment analysis in the brain of WT C57BL/6J recipients transferred with 2D2-Eomes-TWT cells. (E) Proportion of Foxp3+ regulatory T cells in both CD45.1+ and CD45.2+ CD4+ T cell compartments was assessed at 14 dpi in the brain of mice transferred with 2D2-Eomes-TWT and 2D2-Eomes-TKO (n = 8 mice/group). (F and G) (F) Absolute number of CD44high CD4+ T cells and (G) percentage of Foxp3+ CD4+ T cells in spleen, brain, and SC at 14 dpi upon active immunization of Eomes-TWT and Eomes-TKO mice (n = 7 and 3 mice/group). (H and I) (H) Percentage and (I) absolute number of 2D2-TWT, 2D2-Eomes-TKO, and 2D2-Tbet-TKO cells were analyzed at 14 dpi in dLN, spleen, brain, and SC (n = 6, 5, and 5 mice, respectively). Data are a pool of two experiments (E) or representative of at least two independent experiments. Error bars = SEM; P values (Mann–Whitney U test) or P values (two-way ANOVA with Bonferroni correction for clinical scores)—**P < 0.01, *P < 0.05.
Figure 1.
Eomes is required for pathogenic CD4+ T cells functions during neuroinflammation. (A) 200,000 naïve CD4+ T cells from 2D2-Eomes-TWT or 2D2-Eomes-TKO mice were injected into WT C57BL/6J recipient mice prior to immunization (n = 23 and 20 mice per group, respectively) and clinical scores were evaluated and cumulative and maximal scores were determined. (B) 2D2-Eomes-TWT and 2D2-Eomes-TKO mice were immunized with MOG35–55 peptide emulsified in CFA. At 7 dpi, cells from dLN and spleen were collected and stimulated with MOG35–55 in the presence of anti-IFN-γ mAb and IL-23. CD4+ T cells were then purified, and 800,000 cells were injected i.v. into C57BL/6J recipients (n = 8 mice per group). Clinical scores were then evaluated, and cumulative and maximal scores were determined. (C) Naïve CD45.1+ cells purified from 2D2-Eomes-TWT or 2D2-Eomes-TKO mice were injected into WT C57BL/6J CD45.2 recipient mice (n = 4–6 mice per group) prior to immunization with MOG35–55 peptide and percentage of CD45.1+ cells among CD4+ T cells was assessed in both dLN and spleen (SPL) at 8 dpi. (D) Absolute numbers of 2D2 cells (CD45.1) and endogenous CD4+ T cells (CD45.2) were assessed in both dLN and spleen at 8 dpi (n = 6 mice/group). (E and F) (E) Ki67 and (F) activation marker expression were analyzed in both CD45.1+ and CD45.2+ CD4+ T cells from mice injected with either 2D2-Eomes-TWT and 2D2-Eomes-TKO cells at 8 dpi (n = 4 or 5 and 5 mice, respectively). (G) Intracellular staining of IFN-γ, IL-17, and GM-CSF expression by 2D2-Eomes-TWT and 2D2-Eomes-TKO CD45.1+ T cells from the dLN was assessed at 8 dpi after 48 h of ex vivo restimulation with MOG35–55 peptide (n = 10 and 9 mice/group). (H) Percentage of Foxp3+ CD4+ T cells was assessed in both CD45.1+ and CD45.2+ compartments in dLN and spleen at 8 dpi (n = 4 and 5 mice). (I) Naïve CD4+ T cells from 2D2-Eomes-TWT and 2D2-Eomes-TKO mice were injected into C57BL/6 mice prior to immunization (n = 6 mice/group), and percentages of CD45.1+ 2D2-Eomes-TWT or 2D2-Eomes-TKO cells were analyzed at 14 dpi in dLN, cervical lymph nodes (cLN), spleen, brain, and spinal cord. (J and K) (J) Absolute number of CD45.1+ 2D2-Eomes-TWT or 2D2-Eomes-TKO cells or (K) endogenous CD45.2+ CD4+ T cells was assessed at 14 dpi in the brain and SC of mice treated as in I. Data are representative of at least two independent experiments and a pool of two (G) and three independent experiments (A). Error bars = SEM; P values for cumulative and maximal scores (Mann–Whitney U test), P values for clinical scores, cytokine production, and absolute numbers (two-way ANOVA with Bonferroni correction)—****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05. See also Fig. S1.
To address the impact of Eomes deletion on MOG-specific CD4+ T cells during the T cell priming phase of EAE, we used the model of naïve 2D2 transfer as described in Fig. 1 A. At 7 days post immunization (dpi), the analysis of the proportion, absolute number, and frequency of Ki67-expressing cells in both transferred CD45.1+ cells or endogenous CD45.2+ cells revealed that Eomes deficiency did not affect CD4+ T cell proliferation (Fig. 1, C–E). In addition, both 2D2-Eomes-TWT and 2D2-Eomes-TKO cells and the matched endogenous compartment (CD45.2) showed equivalent expression of activation (Fig. 1 F) and migration (Fig. S1 C) markers, suggesting that early activation events are not affected by Eomes deficiency. We next examined the cytokine production of transferred CD45.1+ 2D2 cells upon in vitro restimulation with MOG35–55 peptide. 2D2-Eomes-TKO cells produced significantly less IFN-γ compared with control 2D2 cells, while their production of IL-17 and GM-CSF remained unaffected (Fig. 1 G). Moreover, we evaluated Treg frequency in both transferred 2D2 (CD45.1) and endogenous CD4+ T cells (CD45.2). Our results suggest that Eomes deletion does not impact Foxp3 induction or differentiation of transferred cells or accumulation of endogenous Foxp3+ CD45.2 CD4+ T cells since proportions of Treg remain comparable in each group in both draining lymph nodes (dLN) and spleen (Fig. 1 H).
We next investigated the role of Eomes in MOG-specific CD4+ T cells at a later stage of disease development (14 dpi) in WT mice injected with either 2D2-Eomes-TKO or 2D2-Eomes-TWT CD45.1+ cells as in Fig. 1 A, analyzing both CD45.1 and CD45.2 compartments (Fig. S1 D). At this time point, the vast majority of 2D2-Eomes-TWT cells had migrated into the CNS and were almost undetectable in peripheral lymphoid tissues (Fig. 1 I). When compared with 2D2-Eomes-TWT cells, the proportion and absolute number of 2D2-Eomes-TKO cells were significantly lower in both brain and spinal cord (SC) of immunized mice, and this was not associated with reciprocal 2D2 accumulation in the periphery, suggesting that Eomes deletion did not alter T cell trafficking (Fig. 1, I and J). Besides, decreased Eomes-deficient 2D2 cell infiltration was accompanied by a decreased number of endogenous CD45.2 CD4+ T cells in the brain (Fig. 1 K) while the proportion of Treg in both CD45.1 and CD45.2 compartments was equivalent in mice transferred with Eomes-deficient 2D2 cells or controlled 2D2, excluding any impact of Eomes deletion in Treg accumulation or differentiation (Fig. S1 E). To further address the role of Eomes in polyclonal CD4+ T cells, we measured absolute numbers of CD44high CD4+ T cells upon active immunization of Eomes-TKO and Eomes-TWT mice. As observed in the 2D2 transfer model, Eomes-TKO mice exhibited decreased CD4+ T cell infiltration in both brain and SC (Fig. S1 F) while the Treg compartment was not impacted (Fig. S1 G). Given the redundant roles of Eomes and T-bet, we also analyzed the behavior of 2D2 cells deficient for T-bet (purified from 2D2-T-betfl/fl CD4-Cre+ mice) upon transfer into WT mice that were subsequently immunized. Proportion and absolute number of 2D2-Tbet-TKO cells at 14 dpi were comparable with those of 2D2-TWT cells, both in periphery (dLN and spleen) and CNS (brain and SC), highlighting a specific role of Eomes in CD4+ T cell persistence (Fig. S1, H and I). Together, these results show that Eomes is mostly dispensable for autoreactive CD4+ T cell priming and proliferation but is required for the CNS persistence of effector CD4+ T cells during EAE.
Eomes expression is restricted to a fraction of highly activated effector CD4+ T cells that accumulate in the inflamed CNS
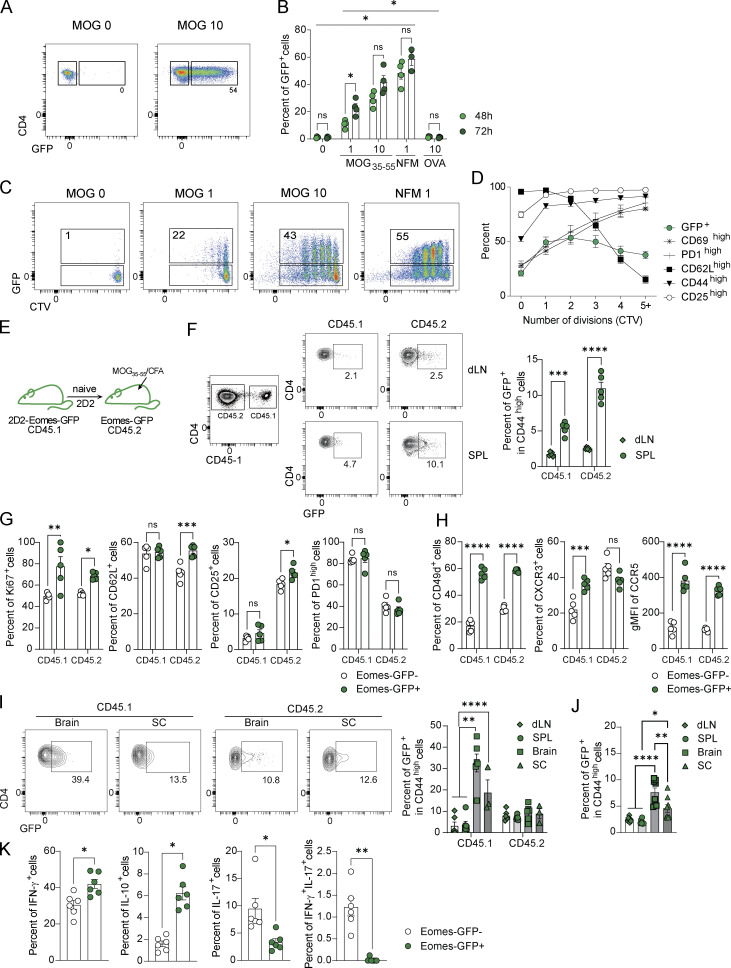
To study how Eomes expression is regulated in CD4+ T cells, we used polyclonal or 2D2 CD4+ T cells originating from Eomes-GFP reporter mice to track Eomes according to GFP expression. In vitro stimulation of 2D2 cells with antigen-presenting cells (APCs) loaded with irrelevant ovalbumin peptide (OVA323–339) failed to induce Eomes expression, whereas a dose-dependent upregulation of Eomes was observed when 2D2 CD4+ T cells were stimulated with APCs loaded with MOG35–55 or the high-affinity neurofilament medium (NFM)15–35 peptide (Krishnamoorthy et al., 2009; Fig. 2, A and B). Moreover, assessing the dynamics of Eomes expression and activation markers alongside cell division, we found that only a fraction of divided and activated 2D2 cells actually expressed Eomes (Fig. 2, C and D). Enhanced Eomes expression was also observed using polyclonal GFP+ CD4+ T cells stimulated with increasing doses of anti-CD3 (Fig. S2 A) or anti-CD28 mAbs (Fig. S2 B), with heightened expression over time (Fig. S2 C) and a similar cell division profile (Fig. S2 D). Together, these results indicate that Eomes expression is induced in a subset of activated CD4+ T cells in a TCR-dependent manner proportional to signal strength and duration.
Figure 2.
Eomes expression delineates a population of highly activated CD4+ T cells that accumulate in the CNS during neuroinflammation. (A) Representative dot plots of CD4 versus GFP expression of naïve 2D2-Eomes-GFP cells unstimulated (left) or stimulated for 72 h in vitro with APCs loaded with 10 μg/ml of MOG35–55 peptide (right). (B) Percentages of GFP-expressing cells of naïve 2D2-Eomes-GFP cells stimulated in vitro with APCs loaded with MOG35–55 (1 or 10 μg/ml), NFM (1 μg/ml), or OVA (10 μg/ml) and was measured after 48 and 72 h of stimulation (n = 4 mice). (C) Representative dot plots of CTV staining according to GFP expression in 2D2 CD4+ T cells stimulated in vitro for 72 h with MOG35–55- or NFM-loaded APCs. (D) GFP expression compared with activation marker expression against the number of cell divisions in 2D2 CD4+ T cells (n = 4 mice). (E) Naïve 2D2-Eomes-GFP CD45.1+ cells were injected into Eomes-GFP CD45.2 recipient mice prior to immunization with MOG35–55 peptide in CFA. (F) Representative dot plots of CD4 versus GFP (left) and percentages of GFP-expressing cells (right) gated on CD44high CD45.1+ and CD44hi CD45.2+ CD4+ T cells, in dLN and spleen (SPL) of immunized mice at 8 dpi (n = 5 mice). (G and H) Expression of (G) Ki67, activation markers, and (H) migratory markers in CD44high CD45.1+ and CD44hi CD45.2+ CD4+ T cells in the spleen at 8 dpi (n = 5 mice). (I) Percentages of GFP+ cells were analyzed in CD44hi CD45.1+ and CD44hi CD45.2+ CD4+ T cells at 14 dpi in dLN, spleen, brain, and spinal cord (n = 6 mice per group, and for SC, cells were pooled to have three independent samples). (J) Percentage of GFP+ cells was analyzed in CD44high CD4+ T cells in a model of active EAE at 14 dpi in dLN, spleen, brain, and SC (n = 8 mice). (K) Intracellular expression of IFN-γ, IL-10, IL-17, and IFN-γ/IL-17 was assessed in Eomes-GFP− and Eomes-GFP+ cells from the brain after an overnight restimulation with 10 µg/ml of MOG35–55 peptide (n = 6 mice). Data are representative of at least two independent experiments. Error bars = SEM; P values for B and F–J (two-way ANOVA with Bonferroni correction), P values in K (Mann–Whitney U test)—****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05. See also Fig. S2.
Figure S2.
Eomes expression is induced in a TCR/CD28-dependent manner proportionally to signal strength and duration and Eomes+ CD4+ T cells accumulate in the CNS over the course of inflammation. (A) GFP expression in CTV-labeled Eomes-GFP CD4+ T cells stimulated for 48 h with 1 µg/ml of anti-CD28 and increasing doses of anti-CD3 mAb. (B) Same as A but with 0.5 µg/ml of anti-CD3 and increasing doses of anti-CD28 mAb (n = 5 mice). (C) GFP expression in Eomes-GFP CD4+ T cells stimulated for 24, 48, and 72 h with 2 µg/ml of anti-CD3 and 1 µg/ml of anti-CD28 mAbs (n = 3 mice). (D) GFP expression compared to activation marker expression against number of cell divisions in CD4+ T cells was assessed in Eomes-GFP CD4+ T cells stimulated in vitro for 48 h (n = 5 mice). (E) Representative gating strategy showing GFP expression in CD45.1+ and CD45.2+ compartment in the brain of WT C57BL/6J recipients transferred with 2D2-Eomes-TWT cells. (F) Ki67 and activation marker expressions were analyzed in GFP− and GFP+ CD45.1+ or CD45.2+ CD44high CD4+ T cell compartments in the brain at 14 dpi (n = 6 mice). (G) CD107a expression was assessed in Eomes-GFP− and Eomes-GFP+ CD44high CD4+ T cells from the brain at 14 dpi upon active immunization of Eomes-GFP mice and restimulation ex vivo overnight with 10 µg/ml of MOG35–55 peptide (n = 6 mice). (H) Percentage of injected cells in NI mice in blood, spleen, dLN, and cLN 8 dpi (n = 4 mice). (I and J) Percentage and absolute number of 2D2 injected cells were analyzed at 5, 8, and 14 dpi (n = 5, 6, and 6 mice, respectively) in (I) cLN and (J) blood. (K and L) Proliferation rates were assessed at (K) 5 dpi or (L) 8 and 14 dpi in spleen (SPL), dLN, brain, cLN, and blood of immunized mice. Data are representative of two independent experiments. Error bars = SEM; P values in G (Mann–Whitney U test) and other P values (two-way ANOVA with Bonferroni correction)—****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
To further understand Eomes regulation in CD4+ T cells over the course of EAE, 2D2-Eomes-GFP (CD45.1+) CD4+ T cells were transferred into Eomes-GFP recipient mice (CD45.2+) that were subsequently immunized with MOG35–55 peptide (Fig. 2 E). We next analyzed the frequency of GFP+ CD44high CD4+ T cells in both MOG-specific (CD45.1+) and polyclonal CD4+ (CD45.2+) T cells (Fig. S2 E). While GFP expression was mostly undetectable in non-immunized (NI) mice (not shown), we observed a slight increase in the proportion of GFP+ cells at 8 dpi in both CD45.1+ and CD45.2+ compartments with preferential localization to spleen over dLN (Fig. 2 F). Moreover, while GFP+ CD44high CD4+ T cells exhibited slightly augmented proliferation as shown by the increased expression of Ki67 in both CD45.1+ and CD45.2+ compartments, Eomes expression did not drastically impact cell activation as shown by CD62L, CD25, or PD1 expressions compared with GFP− cells (Fig. 2 G). By contrast, GFP+ CD44high CD4+ T cells from both CD45.1+ and CD45. 2+ compartments expressed higher levels of CD49d, CXCR3, and CCR5 when compared with GFP− cells suggesting enhanced migratory properties in Eomes-expressing cells (Fig. 2 H).
At a later stage of disease development (14 dpi), we found that, while Eomes expression did not drastically impact the phenotype of CNS-infiltrating cells (Fig. S2 F), an accumulation of Eomes-expressing 2D2 cells was found in the CNS with an average of 33% and 18% of 2D2 T cells expressing GFP in the brain and SC, respectively (Fig. 2 I). Such accumulation of GFP+ cells was not observed in polyclonal CD45.2+ cells, suggesting a potential competition between transferred 2D2 and endogenous CD4+ T cells. Therefore, we used the model of active EAE induced upon immunization of Eomes-GFP mice and analyzed the proportion of GFP+ CD44high CD4+ T cells at 14 dpi. We confirmed in polyclonal repertoire that Eomes-expressing CD44high CD4+ T cells accumulated in both brain and SC (Fig. 2 J). Analyses of cytokine production upon active EAE induction showed that IFN-γ expression was increased in Eomes-GFP+ cells. Moreover, Eomes-expressing CD4+ T cells showed enhanced IL-10 production and reduced proportions of IL-17 single-positive and IFN-γ/IL-17 double-positive cells compared with GFP− CD4 T cells (Fig. 2 K). GFP+ CD4+ T cells also exhibited increased CD107a expression suggesting enhanced cytotoxic functions associated with Eomes expression (Fig. S2 G). Hence, Eomes expression is restricted to a fraction of highly activated CD4+ T cells, producing mainly IFN-γ and IL-10, that accumulate in the CNS over the course of EAE.
Eomes supports effector CD4+ T cell survival over the course of EAE
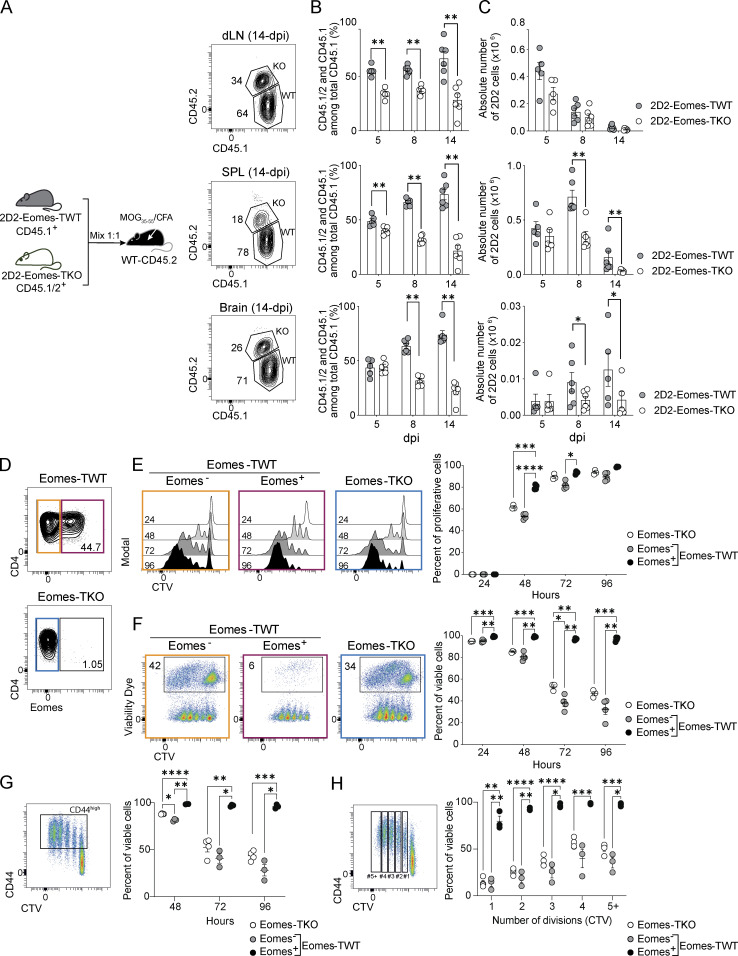
To investigate the mechanisms by which Eomes favors CD4+ T cells accumulation into the CNS, co-transfer experiments were performed using naïve CD4+ T cells from 2D2-Eomes-TWT and 2D2-Eomes-TKO mice expressing CD45.1+ or CD45.1/2+, respectively. The two populations were mixed at a 1:1 ratio and transferred into WT CD45.2 recipients (Fig. 3 A). In the absence of immunization, equal proportions and numbers of 2D2-Eomes-TKO and -TWT cells were found in peripheral lymphoid organs (Fig. S2 H). We then monitored 2D2 cell recovery over time after immunization and found that the proportion and absolute numbers of 2D2-Eomes-TKO cells decreased at 8 and 14 dpi in all organs tested (Fig. 3, B and C; and Fig. S2, I and J). This impaired maintenance of 2D2-Eomes-TKO cells is not the consequence of decreased proliferation, as both 2D2-Eomes-TWT or 2D2-Eomes-TKO cells exhibited similar proliferation as measured by CellTrace Violet (CTV) dilution at 5 dpi and Ki67 staining at 8 and 14 dpi (Fig. S2, K and L).
Figure 3.
Eomes drives the survival of effector CD4+ T cells both in vitro and in vivo during neuroinflammation. (A) Naïve CTV-labeled 2D2-Eomes-TWT CD45.1+ and 2D2-Eomes-TKO CD45.1/2+ cells were coinjected at a 1:1 ratio into C57BL/6J mice subsequently immunized with MOG35–55 peptide in CFA. Representative gating strategy is shown. (B and C) (B) Percentage and (C) absolute number of 2D2-Eomes-TWT CD45.1+ and 2D2-Eomes-TKO CD45.1/2+ among total injected cells in dLN, spleen, and brain at 5, 8, and 14 dpi (n = 5, 6, and 6 mice per timing, respectively). (D) Eomes expression in Eomes-TWT and Eomes-TKO CD4+ T cells stimulated in vitro with anti-CD3 and anti-CD28 mAbs. (E and F) (E) Proliferation and (F) survival of naïve Eomes-TWT or Eomes-TKO CD4+ T cells stimulated in vitro with anti-CD3 and anti-CD28 mAbs for 24, 48, 72, and 96 h (n = 3 and 4 mice/group). (G) Frequency of viable CD44high Eomes-TWT or Eomes-TKO CD4+ T cells stimulated in vitro as in F (n = 4 and 3 mice/group). (H) Frequency of viable Eomes-TWT or Eomes-TKO CD4+ T cells according to cell division upon in vitro stimulation as in F (n = 4 and 3 mice/group). Data are representative of at least two independent experiments. Error bars = SEM; P values (two-way ANOVA with Bonferroni correction)—****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05. See also Fig. S2.
To further test the role of Eomes in effector CD4+ T cell survival, naïve CD4+ T cells from Eomes-TWT or Eomes-TKO mice were stimulated in vitro using anti-CD3 and anti-CD28 mAbs, and CD4+ T cell survival over time was assessed alongside Eomes expression using viability dye incorporation (Fig. 3 D). While cell proliferation remained poorly affected by Eomes deletion (Fig. 3 E), we observed that Eomes+ CD4+ T cells survived better than their Eomes− or Eomes-TKO counterparts. Indeed, while very few dead cells were found among Eomes+ CD4+ T cells even after 96 h of stimulation, WT Eomes− and Eomes-TKO CD4+ T cells exhibited gradually increasing mortality upon activation (Fig. 3 F). In addition, we showed that this enhanced survival is not the consequence of differences in activation or division rate since we also observed enhanced Eomes+ CD4+ T cells survival in CD44high activated cells (Fig. 3 G) or according to cell division (Fig. 3 H). Together, our results demonstrate that Eomes expression enhances survival of effector CD4+ T cells.
Eomes controls the expression of genes involved in mitochondrial metabolism
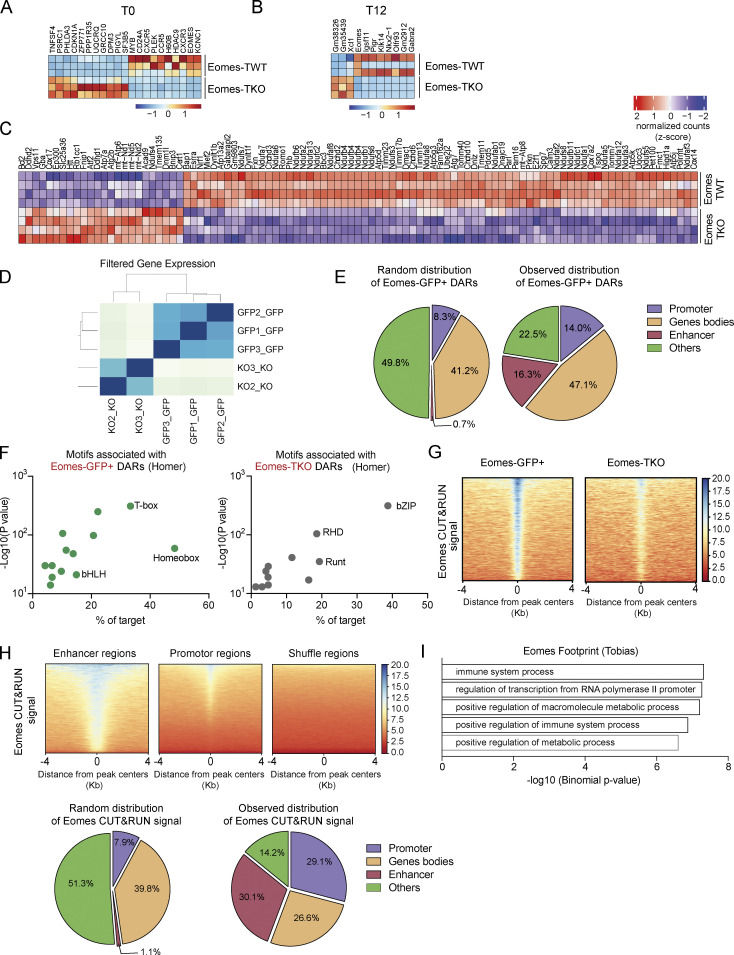
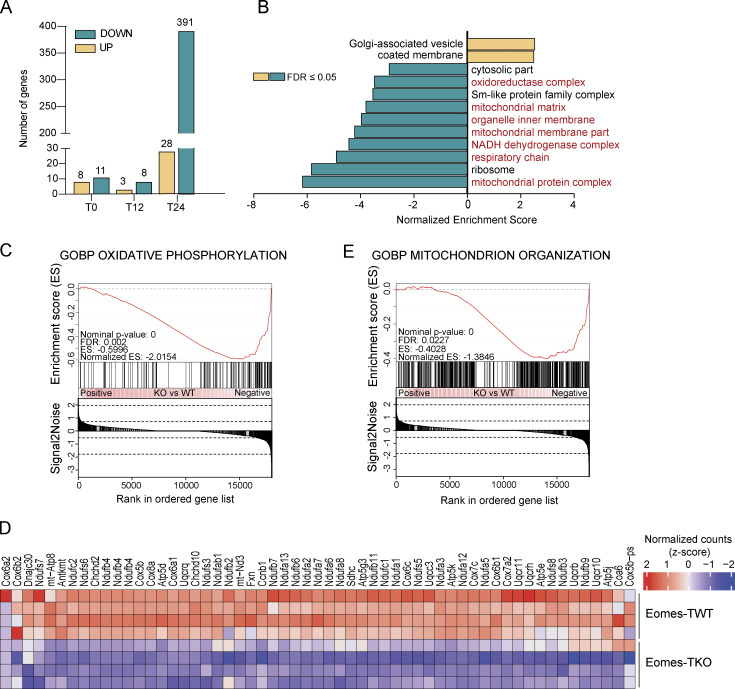
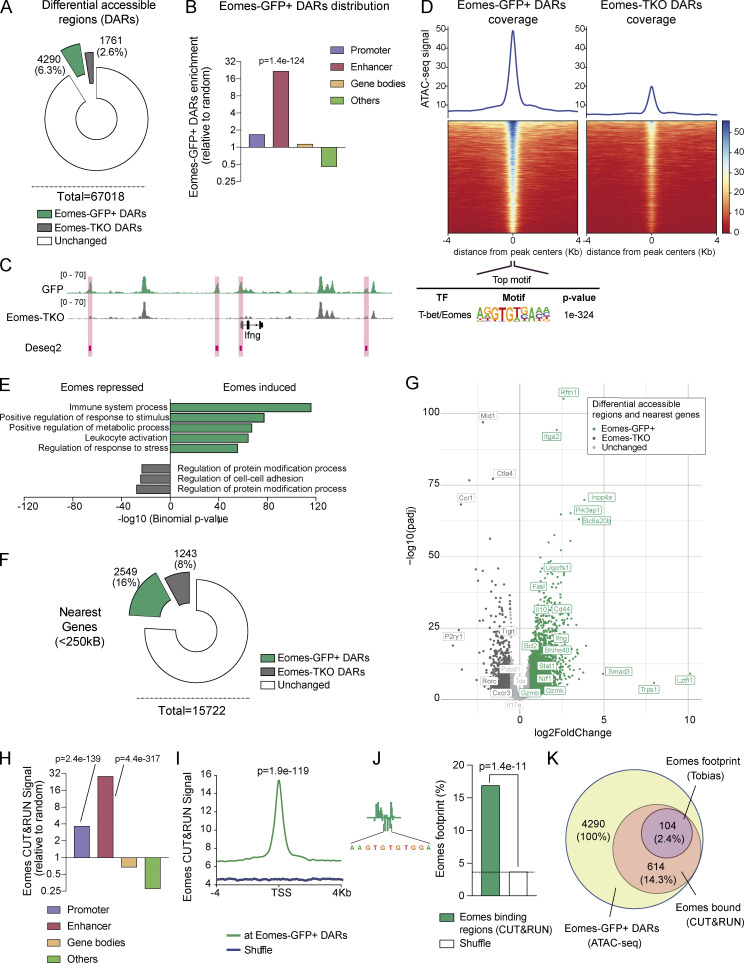
To investigate the molecular mechanisms by which Eomes promotes effector CD4+ T cell survival, we performed RNA sequencing (RNA-seq) analysis using CD4+ T cells from Eomes-TKO and Eomes-TWT mice. Cells were either rested or stimulated for 12 or 24 h with anti-CD3/CD28 mAbs. Very few genes showed differential expression when comparing unstimulated (T0) or early activated CD4+ T cells (T12), illustrating the dispensability of Eomes in naïve CD4+ T cells at steady-state or after short-term activation (Fig. S3, A and B). However, gene expression was drastically altered in Eomes-TKO CD4+ T cells compared with controls after 24 h of stimulation (T24), with 28 upregulated and 391 downregulated genes (Fig. 4 A, adj. P value <0.05). A gene ontology analysis showed that several biological processes related to mitochondrial metabolism were under-represented in Eomes-TKO CD4+ T cell (Fig. 4 B). Ingenuity Pathway Analysis also showed that oxidative phosphorylation (OXPHOS) and mitochondrial dysfunction were the most disrupted pathways in Eomes-TKO CD4+ T cells compared with controls (P values = 6.16e−29 and 1.25e−25, respectively), suggesting that Eomes deletion could alter mitochondria function in CD4+ T cells. Eomes-TKO CD4+ T cells indeed exhibit decreased expression of numerous core signature genes involved in OXPHOS (Fig. 4, C and D) and mitochondrial organization (Fig. 4 E and Fig. S3 C). Thus, regulation of mitochondrial function appears to be a primary target of Eomes-dependent gene expression.
Figure S3.
Multi-omics data analysis uncovers a direct role for Eomes in the regulation of metabolism-associated genes and mitochondrial transcriptional modulators. (A and B) Heatmap of differentially expressed genes between Eomes-TKO and Eomes-TWT CD4+ T cells (A) before (T0) or (B) after stimulation in vitro for 12 h (T12) (n = 3 mice/group). (C) Heatmap of genes involved in mitochondrion organization in Eomes-TKO and Eomes-TWT CD4+ T cells stimulated with anti-CD3 and anti-CD28 mAbs for 24 h. (D) Correlation matrix of Eomes-GFP+ (n = 3) and Eomes-TKO (n = 2) ATAC-seq data. (E) Random and observed genomic distribution of Eomes-GFP+ DARs at the indicated genomic regions. (F) Motif enrichment analyses of Eomes-GFP+ (left) and Eomes-TKO (right) DARs using Homer. (G) Peak density heatmap of Eomes CUT&RUN signal from Eomes-GFP+ (left) and Eomes-TKO CD4+ T cells (right) in the region of 4 kb surrounding Eomes-GFP+ DARs centers determined by ATAC-seq. (H) Peak density heatmap of Eomes CUT&RUN signal from Eomes-GFP+ CD4+ T cells at enhancer, promoter, or shuffle regions (top). Random and observed genomic distribution of Eomes signals as determined by CUT&RUN (bottom). (I) Pathway enrichment analyses of the 88 genes nearest to regions of Eomes footprint (TOBIAS) overlapping regions of Eomes fixation (CUT&RUN) and Eomes-GFP+ DARs (ATAC-seq).
Figure 4.
Eomes transcriptional program mainly impacts genes related to mitochondrial structure and functions. RNA-seq was performed on Eomes-TKO and Eomes-TWT CD4+ T cells stimulated in vitro at 0 (T0), 12 h (T12) or 24 h (T24) (n = 3, 4, and 4 mice/group, respectively). (A) Number of differentially expressed genes between Eomes-TKO and Eomes-TWT CD4+ T cells at T0, T12, and T24. (B) Representation of differentially expressed pathways at T24 (normalized enrichment score [NES] > 2 or NES less than −2) with pathways in red related to mitochondria metabolism. (C and D) (C) GSEA and (D) heatmap of genes involved in OXPHOS showing the (clustered) genes in the leading-edge subsets. (E) GSEA related to genes involved in mitochondria organization in Eomes-TKO and Eomes-TWT CD4+ T cells stimulated with anti-CD3 and anti-CD28 mAbs for 24 h. See also Fig. S3.
Eomes directly regulates genes involved in mitochondrial metabolism in CD4+ T cells
To further decipher the molecular mechanisms whereby Eomes controls the expression of genes involved in mitochondrial function, we combined assay for transposase accessible chromatin (ATAC)-seq and Cleavage Under Targets & Release Using Nuclease (CUT&RUN) experiments in CD4+ T cells. We first investigated Eomes role in genome accessibility by comparing activated Eomes-GFP+ and Eomes-TKO CD4+ T cells upon in vitro stimulation by ATAC-seq (Fig. S3 D). We identified 6,051 differentially accessible regions (DARs) between Eomes-GFP+ and Eomes-TKO CD4+ T cells (DESeq2, fold change [FC] > 1.5 and Padj < 0.01). The majority of peaks (71%, n = 4,290) showed increased accessibility in Eomes-GFP+ cells (referred to as Eomes-GFP+ DARs) compared with Eomes-KO CD4+ T cells (Fig. 5 A and Table S1). Strikingly, Eomes-GFP+ DARs were highly enriched at enhancers (P = 1.4e−124) and, to a lesser extent, promoter regions (P = 5.3e−14) of activated CD4+ T cells (Fig. 5 B and Fig. S3 E). Among them, we identified four peaks in the Ifng locus, in agreement with the role of Eomes in Ifng transcriptional regulation (Fig. 5 C). Further confirming Eomes role in chromatin remodeling, the canonical T-box motif was the top motif present in Eomes-GFP+ DARs (found in 33% of peaks, P = 1e−324) while this motif was not enriched in Eomes-TKO peaks (Fig. 5 D and Fig. S3 F). To decipher the biological consequences of Eomes binding, we next looked for biological process enrichment of Eomes-GFP+ DAR closest genes using Genomic Regions Enrichment of Annotations Tool (GREAT; McLean et al., 2010). Interestingly, the two main enriched pathways were “Immune system process” and “Positive regulation of metabolic process,” further supporting the role of Eomes in metabolic regulation of CD4+ T cells (Fig. 5 E). Among Eomes-GFP+ DAR closest genes associated with immune system, we identified Ifng pathway-associated genes (ifng, igfgr, and stat1), but also genes involved in cytotoxicity (gzmK, gzmB, and gzmL or fasL). Genes associated with metabolic regulation included several transporters, enzymes, and also genes regulating mitochondrial structure and function, in particular Uqcrsf1, Bhlhe40, or Nrf1 (Fig. 5, F and G; and Table S1). Overall, these results established that Eomes confers a unique genome landscape to CD4+ T cells, thus favoring chromatin accessibility at enhancer regions of genes involved in metabolic regulation.
Figure 5.
Eomes favors chromatin accessibility and directly binds to enhancer regions of genes involved in metabolic regulation. (A) Number and percentage of DARs using genome-wide analysis of chromatin accessibility of Eomes-GFP+ (green) and Eomes-TKO CD4+ T cells (gray) as determined by DESeq2 algorithm (FC > 1.5 and Padj < 0.01; n = 3/genotype). (B) Relative enrichment of Eomes-GFP+ DARs at the indicated genomic regions compared with random distribution. (C) Example of track view of the Ifng locus showing ATAC-seq results for Eomes-GFP+ (green track) and Eomes-TKO CD4+ T cells (gray track), with regions of differential accessibility (DESeq2) depicted in pink. (D) Peak density heatmap of ATAC-seq signal obtained from Eomes-GFP+ (Eomes-GFP+ DARs) or Eomes-TKO CD4+ T cells (Eomes-TKO DARs) in regions of 4 kb surrounding peak centers. Motif enrichment analyses associated with Eomes-GFP+ DARs are shown as determined using the Homer algorithm (bottom). (E) Pathway enrichment analysis of genes nearest to Eomes-GFP+ DARs identified by ATAC-seq (GREAT). (F) Number and percentage of genes nearest to Eomes-GFP+ DARs (green) and Eomes-TKO DARs (gray) identified by ATAC-seq within a 250 kb distance from transcription start site (GREAT). (G) Volcano plot of DARs and nearest genes, with Eomes-GFP+ DARs (green dots), Eomes-TKO DARs (dark gray dots), and unchanged (light gray dots) as determined by DESeq2 algorithm. (H) Relative enrichment of Eomes CUT&RUN signal at the indicated genomic elements compare to random distribution. (I) Average plot of Eomes CUT&RUN profiles comparing Eomes binding at Eomes-GFP+ DARs to random regions. (J) Example of aggregated footprint profile of Eomes motif identified by TOBIAS (left) and enrichment plot of Eomes footprint as detected in region of Eomes binding (CUT&RUN) overlapping Eomes-GFP+ DARs or shuffle regions. (K) Venn diagram showing the overlap between regions of Eomes footprint detection (TOBIAS) within regions of Eomes fixation (CUT&RUN) among regions of open chromatin in Eomes-GFP+ CD4+ T cells (ATAC-seq). P values (Pearson’s chi-square test). Also refer to Fig. S3.
To identify Eomes direct target genes, we next mapped the genomic occupancy of Eomes in CD4+ T cells by CUT&RUN assay comparing stimulated CD4+ T cells from Eomes-GFP+ and Eomes-TKO mice (Fig. S3 G and Table S2). In agreement with our ATAC-seq results, Eomes binding sites were preferentially found at enhancer regions (Fig. 5 H and Fig. S3 H) and were strongly enriched at Eomes-GFP+ DARs compared with random distribution (P = 1.9e−119, Fig. 5 I). Overall, 14.3% (614 regions) of Eomes-GFP+ DARs exhibited Eomes binding as defined by CUT&RUN. We next looked for biological process enrichment for Eomes binding site closest genes. We identified two highly enriched pathways: “Immune system process” (P = 4.40e−240) and “Positive regulation of cellular metabolic process” (P = 2.26e−152). To strengthen the identification of direct Eomes binding sites and associated genes, we performed footprinting analysis at Eomes-GFP+ DARs overlapping Eomes binding sites (CUT&RUN) using the TOBIAS tool (Fig. 5 J; Bentsen et al., 2020). These three combined approaches allowed us to validate with high confidence the direct fixation of Eomes in CD4+ T cells to 104 regions associated with 88 genes related with “Immune system process” (n = 23 genes, P = 4.8e−8) and “Positive regulation of metabolic process” (n = 34, P = 2.4e−7; Fig. 5 K, Fig. S3 I, and Table S3). Among these last high-confident set of 88 genes, we identified Uqcrfs1, which encodes an essential subunit of mitochondrial complex, and also Bhlhe40 and Nrf1, two transcription factors known to control the expression of mitochondrial complex genes (Gleyzer et al., 2005; Scarpulla, 2008a). Together, these results strongly support a direct role for Eomes in the regulation of CD4+ T cell metabolism either through direct binding to metabolism-associated genes or via the regulation of mitochondrial transcriptional modulators.
Eomes regulates mitochondrial architecture and functions, thereby promoting effector CD4+ T cell survival
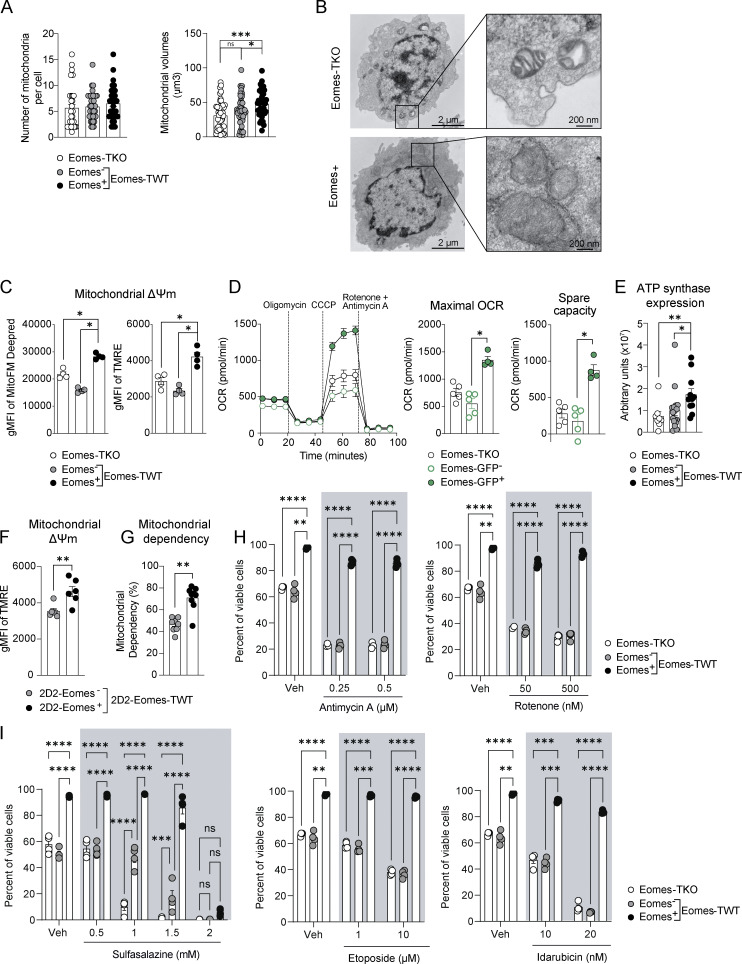
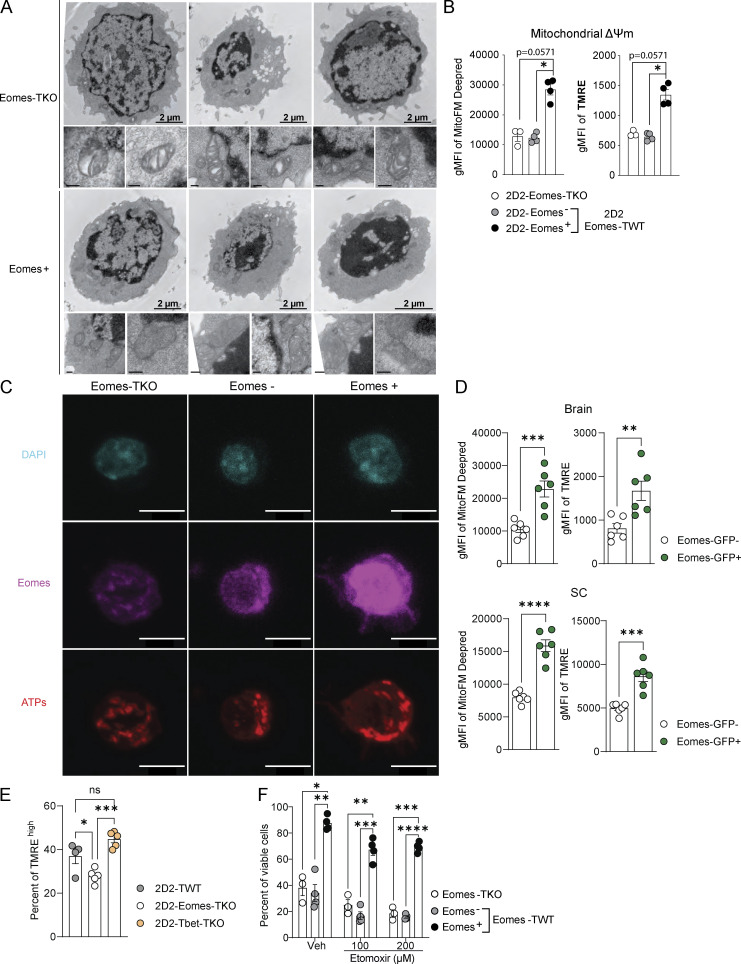
We next addressed whether Eomes-induced transcriptomic and epigenetic changes impacted mitochondrial structure or function. We first examined whether Eomes expression is associated with modifications of mitochondrial morphology. While Eomes expression did not impact the overall number of mitochondria per cell, TOMM20 staining showed that Eomes+ cells had larger mitochondria than Eomes-TKO cells (Fig. 6 A). Moreover, electron microscopic analysis of mitochondria ultrastructure showed that Eomes-GFP+ CD4+ T cells exhibited lamellar cristae morphology and a well-organized and polarized mitochondrial network, while Eomes-TKO cells had isolated and unpolarized mitochondria with aberrant and disorganized cristae (Fig. 6 B and Fig. S4 A). We then assessed whether mitochondrial functions were altered upon Eomes deletion in CD4+ T cells. After in vitro stimulation of polyclonal CD4+ T cells, we used MitoTracker Deep Red FM and TMRE staining to show that Eomes expression correlates with increased mitochondrial membrane potential (ΔΨm) compared with WT Eomes− or Eomes-TKO CD4+ T cells (Fig. 6 C). Activated 2D2-Eomes+ cells also exhibited enhanced ΔΨm as compared with their Eomes-negative and -deficient counterparts (Fig. S4 B). We then used a Seahorse bioanalyzer to measure mitochondrial respiration in Eomes-TKO and Eomes-GFP CD4+ T cells, sorted into Eomes-GFP+ and Eomes-GFP− cells based on GFP expression. Upon polyclonal in vitro stimulation, Eomes-GFP+ CD4+ T cells showed enhanced maximal oxygen consumption rate (OCR) and spare respiratory capacity (SRC) but similar basal OCR compared with Eomes-GFP− CD4+ T cells (Fig. 6 D). This demonstrates that Eomes expression in CD4+ T cells is associated with increased mitochondrial respiratory capacity. Furthermore, the RNA-seq experiment revealed that several ATP synthase subunits were under-represented in Eomes-TKO CD4+ T cells as shown in Fig. 5 D. Elevated expression of ATP synthase associated with Eomes expression was further confirmed at the protein level by quantitative confocal imaging (Fig. 6 E and Fig. S4 C), also suggesting increased respiration efficiency.
Figure 6.
Eomes confers increased mitochondrial respiratory capacities of CD4+ T cells and enhanced their survival abilities under stress conditions. (A) Number and volume of mitochondria were determined in Eomes-TKO, Eomes− and Eomes+ CD4+ T cells by TOMM20 staining and confocal fluorescence microcopy after anti-CD3 and anti-CD28 mAbs stimulation during 48 h. (B) Mitochondrial cristae architecture was imaged using transmission electron microscopy (TEM) in Eomes-TKO and Eomes+ CD4+ T cells. Scale bars represent 2 µm (left) and 200 nm (right). (C) Mitochondrial membrane potential (ΔΨm) of Eomes-TKO, Eomes−, and Eomes+ CD4+ T cells stimulated in vitro for 48 h using MitoFM DeepRed and TMRE stainings (n = 4 mice/group). (D) OCR was assessed in Eomes-TKO, Eomes−, and Eomes+ cells stimulated in vitro for 48 h (left). Maximal OCR and SRC were also determined (right) (each point represents an experiment replicate from a pool of three or six mice/group for Eomes-TKO and Eomes-GFP, respectively). (E) ATP synthase expression was determined using confocal fluorescence microcopy. (F and G) (F) ΔΨm and (G) mitochondrial dependency were assessed in 2D2-Eomes− and 2D2-Eomes+ CD4+ T cells purified from the brain of immunized mice (n = 6 and 8 mice for F and G, respectively). (H and I) Eomes-TKO and Eomes-TWT CD4+ T cells were stimulated during 48 h with anti-CD3 and anti-CD28 mAbs in presence of either (H) antimycin A and rotenone or (I) sulfasalazine, etoposide, and idarubicin (n = 4 mice/group). Data are a pool of two (E), three (A), or representative of at least two independent experiments. Error bars = SEM; P values in F and G (Mann–Whitney U test), other P values (two-way ANOVA with Bonferroni correction)—****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05. See also Fig. S4.
Figure S4.
Eomes deletion is associated with disorganization of mitochondrial cristae structure, decreased ATP synthase expression, and enhanced sensitivity to stress-induced cell death. (A) Mitochondrial cristae architecture was imaged and analyzed by TEM in Eomes-TKO and Eomes+ CD4+ T cells. Scale bars represent 2 µm (cell scale) and 200 nm (mitochondria scale). (B) Mitochondrial membrane potential (ΔΨm) was assessed in 2D2-Eomes-TKO, Eomes−, and Eomes+ CD4+ T cells stimulated in vitro with 1 µg/ml MOG35–55 loaded-APCs for 48 h using MitoFM DeepRed and TMRE staining (n = 3 and 4 mice/group). (C) Representative images of DAPI (blue), Eomes (pink), and ATP synthase (red) stainings in Eomes-TKO, Eomes−, and Eomes+ CD4+ T cells using confocal microscopy after 48 h of in vitro stimulation with anti-CD3 and anti-CD28 mAbs. Scale bars represent 7 µm. (D) MitoFM Deepred and TMRE stainings were analyzed in CD44high CD4+ T cells infiltrating the brain and SC upon active EAE immunization of Eomes-GFP reporter mice (n = 6 mice). (E) Mitochondrial membrane potential (ΔΨm) of 2D2-TWT, 2D2 Eomes-TKO, and 2D2-Tbet-TKO was assessed at 8 dpi in dLN using TMRE (n = 4, 5, and 5 mice, respectively). (F) Eomes-TKO and Eomes-TWT CD4+ T cells were stimulated during 48 h with anti-CD3 and anti-CD28 mAbs followed by 48 h of stimulation in the presence of high doses of etomoxir, and viable cells were identified using viability dye incorporation (n = 3 and 4 mice/group). Data are representative of two independent experiments. Error bars = SEM; P values (Mann–Whitney U test) and P values (two-way ANOVA with Bonferroni correction)—****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
Next, we tested the significance of these results in the in vivo EAE model. Because 2D2-Eomes-TKO cells failed to survive in the inflamed CNS, we compared Eomes+ versus Eomes− 2D2-Eomes-TWT cells extracted from the same CNS of immunized mice at 12 dpi. In line with in vitro results, expression of Eomes by CNS-infiltrating CD4+ T cells was associated with enhanced mitochondrial capacities as measured by ΔΨm-sensitive dyes (Fig. 6 F). Moreover, using the SCENITH technique (Argüello et al., 2020), we confirmed that CNS-infiltrating Eomes+ 2D2 cells displayed increased mitochondrial dependency compared with Eomes− 2D2 cells from the same CNS (Fig. 6 G). Enhanced mitochondrial activity associated with Eomes expression was also observed in polyclonal CD44high CD4+ T cells infiltrating the brain and SC upon active EAE immunization of Eomes-GFP reporter mice (Fig. S4 D). Moreover, to analyze whether the regulation of mitochondrial functions was specific to Eomes rather than a function shared by “T-box” TF, we next assessed mitochondrial activity of 2D2-Tbet-TKO cells upon transfer into WT recipient mice that were subsequently immunized. Contrary to Eomes deletion, T-bet deficiency was not associated with reduced mitochondrial activity, highlighting a specific role of Eomes in mitochondrial function regulation (Fig. S4 E).
We next assessed how enhanced mitochondrial respiratory capacity would affect CD4+ T cell survival and their ability to withstand mitochondrial stress. We stimulated CD4+ T cells in the presence of increasing doses of antimycin A and rotenone, two inhibitors targeting respectively the complex III and I of the mitochondrial electron transport chain (ETC). Following treatment, we observed a dose-dependent induction of cell death in Eomes-TKO and Eomes−, while Eomes+ CD4+ T cells were resistant to ETC complexes inhibition (Fig. 6 H). Enhanced survival of Eomes+ CD4+ T cells was also observed upon stimulation with high doses of Etomoxir, which inhibits both fatty acid mitochondrial transporter CPT1a and adenine nucleotide translocator (ANT; Fig. S4 F). To further investigate whether increased mitochondrial metabolism in Eomes+ CD4+ T cells could explain their increased survival ability in inflamed tissues characterized by enhanced oxidative stress, we assessed cell survival under oxidative stress induced by sulfasalazine (antioxidant synthesis inhibitor), etoposide, and idarubicin (topoisomerase II inhibitors) exposures (Fig. 6 I). While Eomes-TKO and Eomes− CD4+ T cells exhibited increased cell death, Eomes+ CD4+ T cells showed enhanced resistance to oxidative stress-induced cell death, further demonstrating the direct link between mitochondrial metabolism and long-term survival of CD4+ T cells under stress conditions. Altogether, these results further demonstrate the role of Eomes in the regulation of mitochondrial capacities both in vitro and in vivo and highlight that enhanced mitochondrial functions determine effector CD4+ T cell survival skills under stress conditions or in inflamed CNS.
EOMES+ CD4+ T cells from healthy donors and MS patients showed enhanced mitochondrial functions
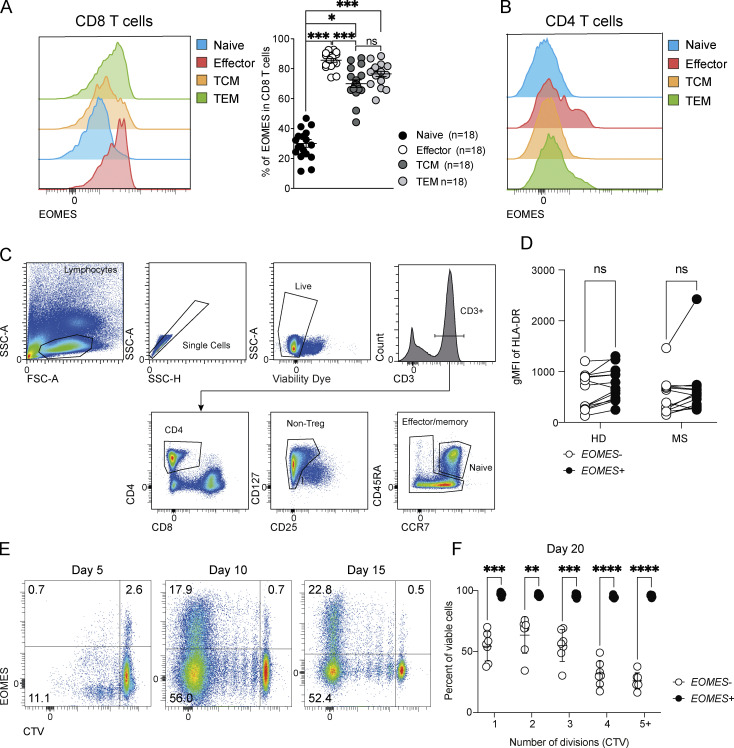
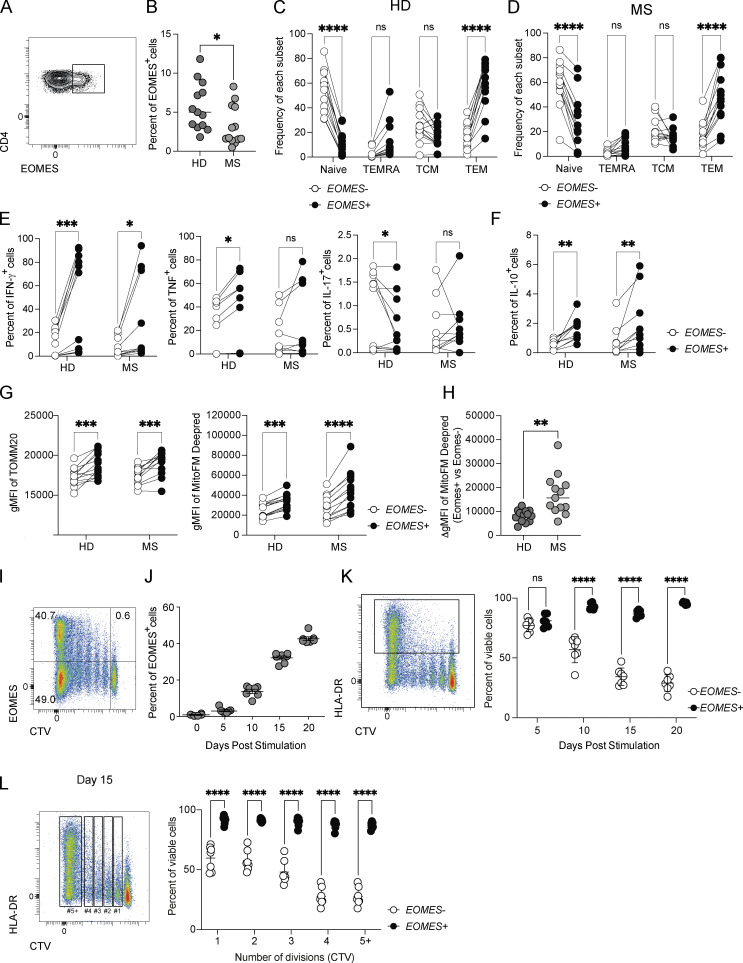
As EOMES was previously identified as a susceptibility gene in inflammatory diseases including MS, RA, and IBD, we next addressed whether similar findings could also be recapitulated in human CD4+ T cells, especially in context of neuroinflammation. To do so, we quantified EOMES expression in CD4+ T cells in peripheral blood of untreated MS patients and sex- and aged-matched healthy donors (HD). While the proportion of EOMES+ cells is relatively low in CD4+ T cells as compared with CD8+ T cells (Fig. S5 A), we detected a significant proportion of EOMES+ CD4+ T cells in peripheral blood mononuclear cells (PBMC) of HD (Fig. 7 A), which was slightly reduced in MS patients (Fig. 7 B). In both HD and MS patients, the frequency of EOMES+ cells was superior in effector memory cells (CCR7−CD45RA−) while decreased in naïve CD4+ T cells (CCR7+CD45RA+; Fig. 7, C and D; and Fig. S5 B). We next analyzed the phenotype and cytokine production of EOMES-expressing CD4+ T cells upon gating within effector/memory CD4+ T cells (Fig. S5 C). HLA-DR (human leukocyte antigen, DR isotype) expression was similar between EOMES+ and EOMES− effector/memory CD4+ T cells, suggesting equivalent activation rate between both populations (Fig. S5 D). Besides, analyses of cytokine production by effector/memory CD4+ T cells upon ex vivo stimulation with PMA/ionomycin showed enhanced expression of IFN-γ and IL-10 in EOMES+ cells compared with EOMES− cells from both HD and MS patients while TNF expression was increased and IL-17 reduced in EOMES+ cells only in CD4+ T cells from HD (Fig. 7, E and F). To confirm the role of EOMES in mitochondrial metabolism, we next evaluated mitochondrial mass and function according to EOMES expression in effector/memory CD4+ T cells from HD and MS patients. As shown in our preclinical model of EAE, EOMES expression was associated with enhanced mitochondrial mass and activity as demonstrated by the increased TOMM20 and MitoFM Deepred stainings (Fig. 7 G), with a more pronounced augmentation of mitochondrial function found in MS patients compared with HD (Fig. 7 H). Altogether, these results confirmed in MS patients that Eomes plays a key role in controlling mitochondrial metabolism in effector/memory CD4+ T cells.
Figure S5.
EOMES expression enhances survival capacity in human CD4+ T cells. (A) Representative histogram plots and frequencies of EOMES expression in naïve (CCR7+CD45RA+), effector (CCR7−CD45RA+), central memory (TCM; CCR7+CD45RA−), or effector memory (TEM; CCR7−CD45RA−) CD8+ T cells from PBMC of HD (n = 18). (B) Representative histogram plot of EOMES expression in naïve (CCR7+CD45RA+), effector (CCR7−CD45RA+), central memory (CCR7+CD45RA−), or effector memory (CCR7−CD45RA−) CD4+ T cells from PBMC of HD. (C) Representative dot plot of the gating strategy used to select effector/memory CD4+ T cells from PBMC of HD. (D) Geometric mean fluorescence intensity (gMFI) of HLA-DR expression in EOMES+ and EOMES− effector/memory CD4+ T cells from PBMC of HD (n = 13) and MS (n = 13) patients. (E) Representative dot plot of EOMES expression according to CTV dilution of naïve CD4+ T cells purified from PBMC of HD and stimulated for 5, 10, and 15 days with coated anti-CD3 and soluble anti-CD28 mAbs (n = 7). (F) Frequency of viable cells according to cell division of CD4+ T cells from HD (n = 7) stimulated as in E for 20 days by viability dye incorporation. Data are representative of at least two independent experiments. Error bars = SEM; P values (two-way ANOVA with Bonferroni correction)—****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05.
Figure 7.
EOMES expression is linked with enhanced mitochondrial function and improves survival capacity of human CD4+ T cells from both HD and MS patients. (A) Representative dot plot of EOMES expression in total CD4+ T cells from PBMC of HD. (B) Frequencies of EOMES expression in total CD4+ T cells from HD (n = 13) and MS (n = 13) patients. (C and D) Frequencies of naïve (CCR7+CD45RA+), effector (TEMRA; CCR7−CD45RA+), central memory (TCM; CCR7+CD45RA−), or effector memory (TEM; CCR7−CD45RA−) within EOMES− and EOMES+ CD4+ T cells from (C) HD (n = 13) or (D) MS (n = 13) patients. (E and F) (E) Intracellular IFN-γ, TNF, IL-17, and (F) IL-10 expression was assessed in EOMES− and EOMES+ effector/memory CD4+ T cells from HD (n = 11) and MS (n = 10) donors after PMA/ionomycin stimulation. (G and H) (G) Mitochondrial mass (TOMM20) and mitochondrial membrane potential (MitoFM Deepred) were assessed in EOMES− and EOMES+ effector/memory CD4+ T cells from HD (n = 13) and MS (n = 13) patients and (H) the ΔgMFI of MitoFM Deepred staining in EOMES+ versus EOMES− was calculated for HD (n = 13) and MS (n = 13) patients. (I and J) (I) Dot plot of EOMES expression according to CTV dilution by CD4+ T cells purified from PBMC of HD (n = 7) and stimulated for 20 days with coated anti-CD3 and soluble anti-CD28 mAbs and (J) frequency of EOMES expression by naïve CD4+ T cells purified from PBMC of HD (n = 7) and stimulated for 5, 10, 15, or 20 days with coated anti-CD3 and soluble anti-CD28 mAbs. (K) Frequency of viable cells in HLA-DR+ CD4+ T cells from HD (n = 7) stimulated as in J for the indicated period by viability dye incorporation. (L) Frequency of viable cells according to cell division in CD4+ T cells from HD (n = 7) stimulated as in J for 15 days by viability dye incorporation. Data are representative of at least two independent experiments. Error bars = SEM; P values (two-way ANOVA with Bonferroni correction)—****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05. See also Fig. S5.
We next evaluated whether, as shown for murine CD4+ T cells, this enhanced mitochondrial metabolism associated with EOMES expression resulted in greater survival upon prolonged in vitro stimulation. Naïve CD4+ T cells were purified from PBMC of HD and stimulated with plate-bound anti-CD3 mAb and soluble anti-CD28 mAb. Stimulation resulted in an upregulation of EOMES expression in CD4+ T cells, with accumulation of EOMES-expressing cells over time (Fig. 7, I and J; and Fig. S5 E). We next evaluated whether the gradual accumulation of EOMES+ CD4+ T cells over time might have resulted from their enhanced survival. Measurement of cell survival over time showed that activated (HLA-DR+) EOMES+ CD4+ T cells exhibited increased survival as compared with EOMES− CD4+ T cells. Indeed, the vast majority of activated CD4+ T cells expressing EOMES showed increased survival even upon 20 days of culture while, at this time point, the vast majority of CD4+ T cells that do not express EOMES were unable to survive (Fig. 7 K). This enhanced survival associated with EOMES expression was not the consequence of the difference in proliferation since we still observed the augmented survival for each cell division (Fig. 7 L and Fig. S5 F). Herein, we demonstrate the significance of our findings in human cells, confirming that EOMES expression confers enhanced survival skills to inflammatory CD4+ T cells associated with increased mitochondrial metabolism.
Discussion
Here, we investigated the cellular and molecular mechanisms by which Eomes favors CD4+ T cell accumulation in inflamed tissues, therefore promoting inflammation. Indeed, our results showed that Eomes-expressing CD4+ T cells migrate and accumulate in the inflamed CNS over the course of EAE. Accordingly, accumulating evidence supports that Eomes plays a broad role in increasing CD4+ T cell accumulation in tissues during chronic inflammation, further suggesting that the breadth and conceptual advance of those findings go beyond neuroinflammation. Indeed, in patients with chronic inflammatory disorders like progressive MS, RA, JIA, and IBD and also in their respective preclinical models, it has been shown that Eomes+ CD4+ T cells accumulate in inflamed tissues. Together, these results strongly suggest a common mechanism involving Eomes in the inflammatory cascade of immunopathology.
The ability of Eomes+ T cells to accumulate in inflamed tissues might result from increased migration, proliferation, or resistance to cell death. Indeed, Eomes drives the expression of several chemokine receptors when expressed by CD8+ T cells or NK cells (Gordon et al., 2012; Zhu et al., 2010). We showed that, during EAE, Eomes-deficient 2D2 cells were fully able to migrate in the CNS and their proliferation capacity was not altered in vitro or in vivo. Rather, our results demonstrate that enrichment of Eomes+ CNS-infiltrating cells is a consequence of the gradual death of Eomes− Th cells. Indeed, Eomes expression increases CD4+ T cell survival both in vitro and in vivo. A similar role for Eomes was reported previously for CD8+ T cells, Eomes being highly expressed in long-lived memory CD8+ T cells. Eomes-TKO mice display altered memory responses, and the few memory CD8+ T cells generated in these mice exhibit impaired long-term persistence (Banerjee et al., 2010), implying a conserved function for Eomes in CD4+ and CD8+ T cell survival. This increased survival of memory CD8+ T cells is tied to their mitochondrial function (van der Windt et al., 2012), with memory CD8+ T cell differentiation and survival both dependent on mitochondrial capacity and respiration (Buck et al., 2016; Champagne et al., 2016). Proper mitochondrial structure and well-organized cristae allow the formation of ETC supercomplexes that maximize OXPHOS and SRC (Cogliati et al., 2013; Mishra et al., 2014), both directly linked to cell longevity (Friedman and Nunnari, 2014; Gomes et al., 2011). Accordingly, we found that the enhanced survival of Eomes+ CD4+ T cells is linked with increased mitochondrial volume, membrane potential, respiratory capacities, and efficient cristae structure. Eomes deletion, meanwhile, drastically impacts mitochondrial function and therefore cell viability. Furthermore, the significance of our findings was confirmed using human lymphocytes. We observed that prolonged in vitro stimulation of naïve CD4+ T cells from HD resulted in enhanced EOMES expression. Moreover, in agreement with what we showed in murine cells, those cells accumulate overtime due to their increased survival capacities coupled to enhanced mitochondrial mass and activity. These results suggest that Eomes might play a conserved role across species in controlling T cell mitochondrial function.
The precise functions of accumulating Eomes+ CD4+ T cells are still ill-defined. Eomes expression can be found in nearly all Th subtypes in both mice and humans, and its functions differ depending on the pathological context (Dejean et al., 2019). For instance, Eomes was shown to contribute to the maintenance of pathogenic Th17-derived Th1 cells during IBD (Mazzoni et al., 2019), Tr1 during graft-versus-host disease (Zhang et al., 2004; Gruarin et al., 2019), memory Th2 in asthma (Endo et al., 2011), or cytotoxic Th9 cells in tumors (Lu et al., 2012). Therefore, each of these studies has proposed a different mode of action of Eomes and a unifying mechanism is still lacking. We revealed here that Eomes favors CD4+ T cell survival by controlling their mitochondrial functions suggesting that, rather than defining a CD4+ T cell lineage, Eomes is a signature of metabolically adapted CD4+ T cells able to survive in a hostile environment such as inflamed tissues.
The molecular mechanisms that regulate mitochondrial function in T cells are not fully understood. It has been shown in CD8+ T cells that CD28 signaling promotes mitochondria remodeling and enhances SRC, and CD8+ T cells primed in the absence of costimulation therefore exhibit decreased survival and impaired memory cell formation (Klein Geltink et al., 2017). In agreement, Eomes expression in CD4+ T cells also depends on CD28 engagement suggesting a common role of Eomes in mitochondrial metabolism regulation downstream CD28. Several proteins have been shown to be required for maximum SRC and OXPHOS efficiency. CD8+ T cells lacking MCJ, a negative regulator of mitochondrial respiration, exhibit enhanced memory responses (Champagne et al., 2016), and T cells from Opa1-deficient mice display decreased OXPHOS activity and SRC associated with decreased long-term survival of memory CD8+ T cells. Moreover, as with Eomes-TKO mice, T cell priming and proliferation of effector cells upon immunization were not altered in Opa1-deficient mice, showing that the initial phases of the immune response are not dependent on mitochondrial dynamics (Buck et al., 2016). Together, these studies show that regulators of increased mitochondrial respiration are associated with enhanced T cell survival, as observed in Eomes+ CD4+ T cells.
Our transcriptomic analysis showed that Eomes deletion resulted in decreased expression of numerous genes involved in mitochondrion structure and function, leading to altered cristae structure organization and decreased mitochondrial respiratory capacity. These results suggest that Eomes is likely regulating another transcriptional regulator involved in mitochondrial function. Our multiomic data combining ATAC-seq, CUT&RUN, and DNA-footprinting analyses identified several new target genes directly regulated by Eomes in CD4+ T cells. Strikingly, 39% of these Eomes targets are involved in cellular metabolic processes confirming the key role of Eomes in CD4+ T cell metabolism regulation. In addition, Bhlhe40 and Nrf1, two key transcriptional regulators of mitochondrial functions, were found to be directly regulated by Eomes in CD4+ T cells. The role of Nrf1 in T cells is mostly unknown. Yet, Nrf1 was shown to control the expression of numerous genes of the respiratory chain complexes (Scarpulla, 2008b) whose expressions were highly reduced in Eomes-deficient CD4+ T cells suggesting that Eomes-mediated regulation of Nrf1 might play a role in the decreased mitochondrial capacity. Bhlhe40, as previously demonstrated in NK cells (Zhang et al., 2021), is also a direct target of Eomes in CD4+ T cells. Several results at the molecular and cellular levels also point to the involvement of the transcription factor Bhlhe40, downstream of Eomes, in the regulation of CD4+ T cell mitochondrial metabolism. Chromatin immunoprecipitation experiments indeed demonstrated that Bhlhe40 directly binds to mitochondrial genes in both CD8+ and CD4+ T cells (Li et al., 2019). As Eomes, Bhlhe40 is upregulated in T cells by activation, with CD28-signaling further enhancing Bhlhe40 expression (Martínez-Llordella et al., 2013). Bhlhe40 regulates the mitochondrial metabolism required for the maintenance and survival of Trm and tumor-infiltrating lymphocytes (TILs; Li et al., 2019). Indeed, TILs with enhanced Bhlhe40 expression had enrichment of OXPHOS genes, and Bhlhe40 deficiency resulted in a decreased expression of a number of electron transport chain genes, as observed in Eomes-GFP+ or Eomes-deficient CD4+ T cells, respectively. Consequently, T cell–specific Bhlhe40 knockout also causes increased sensitivity to cell death, corroborating the phenotype of Eomes-deficient CD4+ T cells both in vitro and during EAE. Taken together, these results show that the Bhlhe40 pathway, downstream of Eomes, is crucial for long-term T cell survival and suggest that this pathway might play key roles during neuroinflammation. In agreement, mice with a T cell–specific deletion of Bhlhe40 were protected from disease in both active and passive EAE models, primarily because Bhlhe40-deficient CD4+ T cells were non-pathogenic (Lin et al., 2016; Martínez-Llordella et al., 2013), as shown here for Eomes-deficient 2D2 cells. Therefore, the Eomes and Bhlhe40 axis might impact the severity and chronicity of inflammation through their regulation of CNS-infiltrating CD4+ T cell mitochondrial metabolism and survival.
Our results show that Eomes expression in CD4+ T cells enhances mitochondrial metabolism, providing a survival advantage that may explain why these cells are preferentially found in inflamed tissues under several pathological conditions (e.g., the CNS during EAE and MS). Metabolic adaptation to the local environment might therefore be mandatory for tissue-homing CD4+ T cells. This is particularly relevant during inflammation, which involves hypoxia and competition for nutrients between inflammatory and resident cells. We show here that Eomes controls the metabolic programs that are required for CD4+ T cells to survive in inflamed tissues. Previous studies using the EAE animal model have indicated that interference with metabolic pathways required for CD4+ T cell early activation, proliferation, or Th17/Treg balance (Berod et al., 2014; Dang et al., 2011; Kornberg et al., 2018) may be an effective therapeutic approach for neuroinflammation. However, these studies mainly addressed peripheral or circulating CD4+ T cell metabolism at early stages of disease development, and the specific metabolic programs required for encephalitogenic CD4+ T cell survival in the inflamed CNS remained undefined. Therefore, suggested therapeutic strategies did not specifically target autoreactive CD4+ T cells and may globally alter immune responses. In this study, we now describe a new mechanism for the control of effector CD4+ T cell longevity through the Eomes-dependent regulation of Bhlhe40 and Nrf1 mitochondrial transcriptional modulators in the context of neuroinflammation. Interference with this pathway could be a promising therapeutic approach to selectively dampen tissue-infiltrating CD4+ T cell pathogenicity while preserving normal global immune responses.
Materials and methods
Mice
C57BL/6J (Janvier), Eomesfl/flCD4-cre (Zhu et al., 2010), Eomes-GFP (Daussy et al., 2014), and 2D2 mice (Bettelli et al., 2003) were maintained in the breeding facility of Centre régional d'exploration fonctionnelle et de ressources expérimentales (Toulouse UMS06) under specific pathogen–free conditions. Mice used for all experiments were littermates and matched for age and sex (both male and female mice were used). Mice for all strains were typically 6–10 wk of age. All animal procedures were conducted in accordance with institutional guidelines on Animal Experimentation and were under a French Ministry of Agriculture license.
PBMC of HD and MS patients
PBMCs were isolated from untreated MS patients in accordance with law no. 2004-806 of August 9, 2004, relating to public health policy. This research (NCT00942214) received a favorable opinion from the Comité de Protection des Personnes Sud Ouest et Outre Mer II and was the subject of a declaration to the Commission Nationale Informatique et Libertés. Age- and sex-matched PBMC from HD were provided by the Institut National de la Santé et de la Recherche Médicale (INSERM) UMR1291 (Toulouse, France). The study on the PBMC from HD subjects was approved by the French South-West & Overseas ethical committee and was registered at the French Ministry of Higher Education and Research (DC-2015-2488). Experiments were performed in agreement with the guidelines of the Declaration of Helsinki. Cryopreservation of PBMCs from patients and healthy volunteers was performed at Institut Toulousain des maladies Infectieuses et Inflammatoires INSERM U1291 (Toulouse, France) within 24 h of blood collection. Cells were stored in liquid nitrogen before use. For each experiment, frozen PBMCs were thawed for 1 min at 37°C in a water bath and then diluted in warm complete RPMI medium. Thawed PBMCs were rested 1 h before staining or purification of naïve CD4+ T cells.
EAE
For active EAE, mice were immunized with 100 µg of MOG35–55 peptide emulsified with CFA containing 1 mg/ml of Mycobacterium tuberculosis (Sigma-Aldrich). 200 ng/ml of pertussis toxin (COGER) was given intravenously on day 0 and day 2 after immunization. For adoptive transfer EAE with polyclonal CD4+ T cells, mice were immunized with 100 µg of MOG35–55 peptide emulsified with CFA containing 1 mg/ml of M. tuberculosis. 10 days after immunization, mice were euthanized, dLNs and spleen were isolated, and cells were restimulated with 20 µg/ml of MOG35–55 peptide, 10 µg/ml of anti-IFN-γ, and 5 ng/ml of IL-23. After 72 h of stimulation, dead cells were removed by Ficoll gradient, CD4+ T cells were purified, and 5 × 106 cells were injected intravenously into recipient mice. For adoptive transfer EAE with 2D2 cells, mice were immunized with 200 µg of MOG35–55 peptide emulsified with CFA containing 1 mg/ml of M. tuberculosis. 7 days after immunization, mice were euthanized, dLNs and spleen were isolated, and cells were restimulated with 20 µg/ml of MOG35–55 peptide, 5 µg/ml of anti-IFN-γ, and 10 ng/ml of IL-23. After 48 h of stimulation, dead cells were removed by Ficoll gradient, CD4+ T cells were purified, and 8 × 105 cells were injected intravenously into sub-lethally irradiated recipient mice. For adoptive transfer of naïve 2D2 cells, 2 × 105 naïve 2D2 cells were injected into WT recipient mice 24 h prior to immunization with 100 µg of MOG35–55 peptide emulsified with CFA containing 1 mg/ml of M. tuberculosis. 200 ng of pertussis toxin were injected twice, at 0 and 2 dpi. Clinical scores were evaluated on a six-stage scale from 0 to 6 as follows: (0) no detectable signs of EAE; (0.5) partial limp tail; (1) complete limp tail; (2) hind limb weakness; (3) severe hind limb weakness; (3.5) unilateral hind limb paralysis; (4) complete hind limb paralysis; (4.5) complete hind limb paralysis and forelimb weakness; (5) total limb paralysis; and (6) death.
Analysis of CNS infiltration and peripheral cytokine production
Mice were anesthetized by intraperitoneal injection of ketamine and xylazine and were then perfused with cold PBS. Brains and SCs were collected separately and were homogenized and digested with collagenase D (2.5 mg/ml; Roche Diagnostics), DNase I (10 μg/ml; Roche), and tosyl-L-lysine chloromethyl ketone (1 μg/ml;Roche) for 30 min at 37°C. Cells were then washed, suspended in 37% Percoll, and layered on 70% Percoll. After a 30-min centrifugation at 2,000 rpm, the mononuclear cells were collected from the interface, washed, and resuspended in culture medium. Isolated cells were counted using a cell counter (Beckman Coulter) and then stained to analyze the presence of different cell populations by flow cytometry.
To analyze cytokine expression by CD4+ T cells, dLN cells and splenocytes were stimulated with different concentrations of MOG35–55 peptide (0, 10, and 100 μg/ml) for 48 h to investigate IFN-γ, IL-17, and GM-CSF production using intracytoplasmic staining.
Murine CD4+ T cell purification and stimulation
Naïve CD4+ T cells were isolated from spleen and lymph nodes by negative selection using the Naïve CD4 T cell Isolation Kit according to the manufacturer’s instructions (Cat: 130-104-453; Miltenyi Biotec). Cells were cultured in RPMI 1640 media supplemented with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin/streptomycin, and 50 μM β-mercaptoethanol in 96-well plates at a seeding concentration of 2 × 105 cells per well. Polyclonal naïve CD4+ T cells were stimulated with 2 μg/ml anti-CD3 antibody (BioLegend) and 1 μg/ml anti-CD28 (BD Biosciences) under non-polarizing conditions. Naïve 2D2 CD4+ T cells were stimulated with irradiated splenocytes loaded with 1 or 10 µg/ml of MOG35–55, 1 µg/ml of NFM, or 10 µg/ml of OVA peptides for 72 h. To analyze CD4+ T cell survival upon mitochondrial complex inhibition or exposure to oxidant stress, CD4+ T cells were stimulated for 48 h with 2 µg/ml of anti-CD3 and 1 µg/ml of anti-CD28 mAbs in the presence of either antimycin A, rotenone, sulfasalazine, etoposide, idarubicin, or etomoxir.
Human CD4+ T cell purification and stimulation
Purification was performed using the naïve CD4+ isolation kit II purchased from Miltenyi Biotec using the manufacturer’s recommendation. 0.2 × 106 naïve CD4+ T cells were stimulated with 2 µg/ml of coated anti-CD3 antibody (BioLegend) with 1 µg/ml of soluble anti-CD28 (BioLegend) in complete RPMI in non-polarizing condition. Medium was fully replaced every 5 days.
Flow cytometry stainings and analyses
Antibody clones, fluorochromes, and suppliers are specified in Table S4. For mitochondrial phenotype, murine and human CD4+ T cells were stained for MitoTracker Deep Red FM (MitoFM Deepred) and TMRE for 20 min at 37°C with 5% CO2. Cell viability was assessed using eBioscience Fixable Viability Dye. Cytokines and transcription factor expression or mitochondrial mass (TOMM20) were measured by intracellular staining using the Foxp3 staining buffer (eBioscience). All samples were acquired and analyzed with the Fortessa flow cytometer (BD Biosciences), FlowJo, and Prism softwares.
Seahorse experiments
After 48 h of stimulation, viable CD4+ T cells were flow-sorted and seeded in wells on 50 µg/ml of poly-D lysine in Seahorse XF Base medium supplemented with sodium/pyruvate (1 mM), glutamine (2 mM), and glucose (25 mM) for 1 h at 37°C, 5% CO2 followed by consecutive addition of oligomycin (1 µM), carbonyl cyanide chlorophenylhydrazone (CCCP; 1.5 µM), antimycin A (1 µM), and rotenone (1 µM). OCR and extracellular acidification rate were measured with XFe24 or XFp Seahorses. To assess dependency on fatty acid oxidation, etomoxir (5 µM) was added between CCCP and antimycin A/rotenone treatments.
RNA-seq sample preparation and analysis
After 0, 12, or 24 h of stimulation, viable CD4+ T cells (in triplicates for T10 or T12 or quadruplicate for T24, pool of two mice per genotype) were flow-sorted. Total RNA was extracted using the RNeasy Micro Kit (Qiagen) and its quality was assessed on a 2100 Bioanalyzer (Agilent Technologies). High-quality RNA (i.e., RNA of integrity number >7) was subsequently used for libraries preparation with TruSeq Stranded mRNA kit protocol according to supplier recommendations (Illumina). Sequencing was carried out on paired-end 150 bp of Illumina HiSeq4000. After trimming of adaptor sequences and removal of low-quality bases (-q value, <15), high-quality reads were aligned to the mouse reference genome mm10 (Genome Reference Consortium) using TopHat version 2.0.5 (Trapnell et al., 2009). Count of the reads mapping to each gene was performed using FeatureCount (package R subread v 2.10.1). Differential gene expression analysis was performed with DESeq2 using the SARTools R package. The significantly (−2 < FC < 2; P.adjust < 0.05) differentially expressed genes were investigated using canonical pathway analyses of Qiagen’s Ingenuity Pathway Analysis and gene set enrichment analysis (GSEA).
ATAC-seq sample preparation and analysis
After 48 h of stimulation, viable CD4+ T cells (in triplicates, pool of two mice for Eomes-GFP+ CD4+ T cells and individual mice for Eomes-TKO) were flow-sorted and ATAC-seq was performed as previously described (Adoue et al., 2019) with minor modifications. Briefly, 50,000 cells were lysed in ice-cold lysis buffer and the transposition reaction was performed using the Tn5 transposase at 37°C for 30 min. DNA was purified using the Qiagen MinElute kit (Qiagen). The libraries were prepared using Tagment DNA enzyme and buffer kits (Illumina), and NEBNext High-Fidelity 2X PCR Master Mix (catalog #M0541S; NEB) with custom sequencing primers (Ackermann et al., 2016; Buenrostro et al., 2013, 2016; Tsompana and Buck, 2014). The libraries were purified twice using AMPure XP beads (Beckman) following a double-sided protocol to remove primer dimers and large fragments. Libraries’ quality was assessed using the NGS High Sensitivity Kit on the Fragment Analyzer (Agilent Technologies). Only high-quality libraries were subsequently equimolarly pooled and sequencing was performed at the Pôle Technologique du CRCT—Plateau de Génomique et Transcriptomique (INSERM-UMR1037, Toulouse, France). The pool of libraries was quantified by quantitative PCR (qPCR) using the KAPA Library Quantification Kit (Roche) to obtain an accurate quantification. Sequencing was then performed on one flowcell of the Illumina NextSeq 550 instrument (Illumina) using the NextSeq 500/550 High Output Kit v2.5 (150 Cycles) and a paired-end 2 × 75 pb strategy. A minimum of 2 × 80 million raw reads were produced per sample.
Adapter trimming and quality controls were performed using Trimgalore v0.6.5. and FastQC v0.11.7. Reads were aligned to the mm10 reference genome by using Bowtie2 v2.2.9. with the splicing alignment feature switched off. The chromatin-accessible peaks were called using Macs2 callpeaks v2.2.7.1 (q value <0.01). FeatureCount (package R subread v 2.10.1) and Deseq2 v 1.36.0 were then used to identify significant differential accessible chromatin regions (Padj < 0.01 and FC > 1.5).
CUT&RUN sample preparation and analysis
After 48 h of stimulation, viable CD4+ T cells (in triplicates, pool of two mice for Eomes-GFP+ CD4+ T cells and individual mice for Eomes-TKO) were flow-sorted and CUT&RUN was performed as previously described (Krzywinska et al., 2022) with some modifications. Briefly, 50,000 cells were harvested and processed with the CUT&RUN Assay Kit (86652; Cell Signaling Technology) following the supplier’s instructions. Eomes monoclonal antibody (Dan11mag, 14-4875-82; Thermo Fisher Scientific) was used at 2 µg. DNA was purified using the Qiagen MinElute kit (Qiagen) and its quality was assessed on a 2100 Bioanalyzer (Agilent Technologies).
Paired-end libraries have been prepared according to Active Motif’s protocol with some adjustments using the Next Gen DNA Library Kit (Active Motif), assuming a starting input of 100 pg of double-stranded DNA. Briefly, DNA first underwent a two-phase reparation. Then, Illumina-compatible adaptors were ligated, allowing the barcoding of the samples with single indices and the addition of molecular identifiers (MIDs) composed of a nine-base random sequence. These MIDs will enable distinguishing PCR duplicates from fragmentation duplicates and the removal of true PCR duplicates from the subsequent sequencing analysis. The libraries were then amplified using 15 cycles of PCR and an additional two-side purification step was finally performed, allowing to obtain 250–1,000 pb fragments. Libraries’ quality was assessed using the NGS High Sensitivity Kit on the Fragment Analyzer (Agilent Technologies) and quantified by qPCR using the KAPA Library Quantification Kit (Roche). Only high-quality libraries were subsequently equimolarly pooled, and sequencing was then performed on one SP lane of the Illumina NovaSeq 6000 instrument (Illumina) using the NovaSeq 6000 SP v1.5 Reagent Kit (300 cycles) and a paired-end 2 × 150 pb strategy. Between 2 × 24 million and 2 × 42 million raw reads were produced per sample. Adapter trimming and quality controls were performed using Trimgalore v0.6.5 and FastQC v0.11.7. Reads were aligned to the mm10 reference genome by using Bowtie2 v2.2.9. with the splicing alignment feature switched off. The peaks were called using Macs2 v2.2.7.1 (q value, <0.01), and Eomes-binding regions were defined as peaks overlapping among Eomes-GFP+ replicates while absent in Eomes-TKO replicates (Bedtools intersect v2.27).
Bioinformatics analyses
R (https://www.R-project.org), SAMtools (Li et al., 2009), and the BEDtools suite v2.22.1 (Quinlan and Hall, 2010) were used to analyze high-throughput sequencing files.
To determine the genome-wide distribution of Eomes-GFP+ DARs or Eomes-binding sites (CUT&RUN), we defined the different genomic regions as follows: gene body coordinates were extracted from assembly GRCm38; promoters were defined as transcription start sites ±2 kb; and enhancers were identified as (Murine_Tcells_Activated_H3K27Ac_48 h_310, GSM2810027) with no overlap with promoters. As a control, we randomly distributed Eomes-GFP+ DARs or Eomes-binding region (CUT&RUN) through the genome using the shuffle sub-command of the BEDtools suite. To measure ATAC-seq and CUT&RUN signal, we used bamCoverage from the deepTools suite (v2.3.4) to generate normalized bedgraph files and Bedtools map to calculate the average signal. Heatmaps were generated by Galaxy Version 3.5.1.0.0. For analysis of motif enrichment, we used Homer annotate Peaks.pl v4.11.1 (Heinz et al., 2010) with default options. For footprinting analysis, TF motifs were downloaded from JASPAR, and TOBIAS algorithm was used as previously described (Bentsen et al., 2020).
De novo detection of TF-binding motif enrichment was performed using the Hypergeometric Optimization of Motif Enrichment (HOMER; Heinz et al., 2010). Biological functions analysis of Eomes-GFP+ DARs or Eomes-binding site (CUT&RUN) coordinates was performed by using GREAT (McLean et al., 2010) with default settings and using the “single nearest gene” option. For Gene Ontology analysis, the “enrichment analysis” tool from the Gene Ontology Consortium was used (http://geneontology.org). We also used GSEA software (http://software.broadinstitute.org/gsea/index.jsp) with default settings except for the “collapse dataset to gene symbols” and “the permutation type,” which were set as “false” and “gene set,” respectively.
SCENITH staining
At 12 dpi, 2 × 106 cells extracted from the CNS were seeded on 96-well plates and incubated for 30 min at 37°C, 5% CO2. Cells were treated for 15 min with control, 2-DG, oligomycin, or a combination of both drugs. Puromycin was added for 45 min at 37°C, 5% CO2. The SCENITH kit containing all reagents was provided by R. Argüello. SCENITH kit instructions were followed concerning the doses of reagents to use. Cells were washed in cold PBS containing 5% FCS and stained with a viability dye followed by surface markers staining, fixation, and permeabilization (Foxp3 staining buffer). Intracellular staining was performed using anti-puromycin monoclonal antibody-AF647 (1:500) contained in the SCENITH kit.
Immunofluorescence microscopy
After 48 h of stimulation, CD4+ T cells were placed on poly-L-lysine coated coverslips for 5 min at room temperature (RT). Cells were fixed for 30 min in 4% paraformaldehyde and permeabilized for 10 min with 0.2% Triton X-100 at RT. Cells were then incubated in a blocking buffer (PBS + 3% bovine serum albumine and 5% FCS) for 1 h at RT. Cells were then incubated with anti-ATPb (ab14730; Abcam), anti-TOMM20 (ab186735; Abcam), and anti-Eomes (14-4875-82; Thermo Fisher Scientific) for 45 min at RT. Then, cells were washed with 0.1% Triton followed by PBS and incubated with Alexa Fluor 594-conjugated goat anti-rabbit (A11012, 1:500; Invitrogen) and Alexa Fluor 647-conjugated goat anti-mouse (A32728, 1:500; Invitrogen) secondary antibodies and DAPI (564907, 1:100,000; BD). Z-stack images were taken by Leica SP8 confocal microscope and processed using Imaris and ImageJ software.
Electron microscopy
After 48 h of stimulation, viable CD4+ T cells were flow-sorted, rested at 4°C for 2 h, and restimulated for 3 h with anti-CD3 and anti-CD28 mAbs. Cells were then collected and fixed with 2% glutaraldehyde in 0.2 M cacodylate buffer for 1 h at 4°C. Fixed cells were centrifuged at 3,000 g for 10 min and incubated in 2% osmium tetroxide. Fixed samples were dehydrated and embedded in resin. Ultrathin sections were prepared using a microtome (Leica UC7) at 60-nm thickness and stained in 2% aqueous uranyl acetate and lead citrate. All images were then captured with a transmission electron microscope at 80 kV (Hitachi-7700).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 10 software. Mann–Whitney test was used for single comparison between two groups. Paired student t test was used to compare GFP+ and GFP− cells from the same subsets or Eomes+ and Eomes− cells from the same patients. Two-tailed one-way analysis of variance (ANOVA) was used to compare >2 conditions for one group while two-tailed two-way ANOVA was used to compare two conditions across multiple groups. In addition, both one- and two-way ANOVA were corrected for multiple comparisons using Bonferroni correction. All error bars represent the SEM. P values <0.05 were considered statistically significant (****P < 0.001, ***P < 0.005, **P < 0.01, *P < 0.05).
Online supplemental material
Fig. S1 shows the clinical scores of active and passive EAE comparing Eomes-TWT and Eomes-TKO mice, the expression of migratory markers on 2D2-Eomes-TKO and 2D2-Eomes-TWT cells, the gating strategy used to evaluate CNS-infiltrating CD4+ T cells as well as the impact of Eomes deficiency on Treg compartment, and the persistence of 2D2-Tbet-KO at 14 dpi. Fig. S2 shows Eomes expression profile upon polyclonal in vitro stimulation, activation marker expression in Eomes− and Eomes+ CD4+ T cells, and proliferation rate of Eomes-TWT and Eomes-TKO 2D2 cells upon in vivo co-transfer experiment. Fig. S3 shows transcriptomic and epigenetic data comparing Eomes+ versus Eomes-TKO CD4+ T cells. Fig. S4 shows electron microscopic analysis of mitochondria ultrastructure, ATP synthase expression, and mitochondrial activity of in vitro–stimulated 2D2 cells and polyclonal CD4+ T cells infiltrating the CNS upon active EAE immunization. Fig. S5 shows the flow-cytometry gating strategy used for human PBMC as well as Eomes expression profile and survival of Eomes-expressing CD4+ T cells from HD upon prolonged in vitro stimulation. Table S1 lists the differentially accessible regions comparing Eomes-GFP+ and Eomes-TKO CD4+ T cells upon in vitro stimulation by ATAC-seq. Table S2 maps the genomic occupancy of Eomes in CD4+ T cells by CUT&RUN assay comparing stimulated CD4+ T cells from Eomes-GFP+ and Eomes-TKO mice. Table S3 lists the regions of Eomes footprints at Eomes-GFP+ open regions (ATAC) overlapping Eomes binding sites (CUT&RUN) using the TOBIAS tool. Table S4 lists antibodies, clones, fluorochromes, and reagents used with suppliers.
Supplementary Material
lists the differential accessible regions comparing Eomes-GFP+ and Eomes-TKO CD4+ T cells upon in vitro stimulation by ATAC-seq.
maps the genomic occupancy of Eomes in CD4+ T cells by CUT&RUN assay comparing stimulated CD4+ T cells from Eomes-GFP+ and Eomes-TKO mice.
lists the regions of Eomes footprints at Eomes-GFP+ open regions (ATAC) overlapping Eomes binding sites (CUT&RUN) using the TOBIAS tool.
lists antibodies, clones, fluorochromes, reagents used with suppliers.
Acknowledgments
The authors acknowledge the contribution of Institut Toulousain des Maladies Infectieuses et Inflammatoires facilities (Institut National de la Santé et de la Recherche Médicale [INSERM] UMR1291—Centre National de la Recherche Scientifique [CNRS] UMR5051—University Toulouse III), in particular A.-L. Iscache, V. Duplan-Eche, and F.-E. L’Faqihi-Olive at the flow-cytometry facility. We thank the Metatoul platform (MetaToul Core Facility, Institut National des Sciences Appliquées, Laboratoire d’ingénierie des systèmes biologiques et des procédés, F-31077 Toulouse, France), in particular F. Bellvert for metabolic analyses; N. Jabrane-Ferra for her help in electronic microscopy analyses; the personnel of the Centre Régional d’Exploration Fonctionnelle et de Ressources Expérimentales (US006) for animal care; and D. Rozet for administrative assistance. We thank Dr. Daniel Ackerman (Insight Editing London) for editing the manuscript during preparation.
The A.S. Dejean lab is supported by the Agence Nationale de la Recherche (ANR GAMBLER and ANR EMETIC), the Fondation pour le Recherche Médicale (FRM; DEQ20170336727), and the Association pour le Recherche sur la Sclérose en Plaques, and it received institutional grants from INSERM, CNRS, Université Paul Sabatier Toulouse III. E. Joulia is the recipient of a fellowship from the FRM (fourth-year PhD). A. Agesta is the recipient of a fellowship from the La Ligue Contre le Cancer (fourth-year PhD). C. Peillex is the recipient of a fellowship from the Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation.
Author contributions: E. Joulia designed, performed, and analyzed most experiments, with significant contributions by M.F. Michieletto, A. Agesta, C. Peillex, V. Girault, A.-L. Le Dorze, R. Marrocco, and R. Peroceschi. E. Lhuillier performed CUT&RUN libraries, A. Chaubet performed ATAC-seq libraries, M. Lebeurrier analyzed RNA-seq dataset, and N. Hakim analyzed the ATAC-seq and CUT&RUN datasets under the supervision of V. Adoue. F. Bucciarelli was in charge of human PBMC collection and storage. H. El Costa designed and set up flow cytometry panels for human PBMC. R.J. Argüello provided Scenith reagents and critical insights. V. Adoue, A. Saoudi, M. Szelechowski, T. Walzer, and J.-E. Sarry provided essential reagents and conceptual insight. A.S. Dejean conceptualized the study, designed the experiments, acquired funding in collaboration with T. Walzer, and wrote the manuscript with contributions from E. Joulia, M.F. Michieletto, A. Saoudi, V. Adoue, T. Walzer, and J.-E. Sarry.
Data availability
The data underlying Figs. 1, 2, 3, 6, and 7 are available in the published article and its online supplemental material. The data underlying Figs. 4 and 5 related to RNA-seq, ATAC-seq, and CUT&RUN datasets are openly available at the Gene Expression Omnibus under accession no. GSE247863. The following published datasets were used in addition: accession nos. GSE135278 (Li et al., 2019) and GSE166718 (Zhong et al., 2022).
References
- Ackermann, A.M., Wang Z., Schug J., Naji A., and Kaestner K.H.. 2016. Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes. Mol. Metab. 5:233–244. 10.1016/j.molmet.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adoue, V., Binet B., Malbec A., Fourquet J., Romagnoli P., van Meerwijk J.P.M., Amigorena S., and Joffre O.P.. 2019. The histone methyltransferase SETDB1 controls T Helper cell lineage integrity by repressing endogenous retroviruses. Immunity. 50:629–644.e8. 10.1016/j.immuni.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Argüello, R.J., Combes A.J., Char R., Gigan J.P., Baaziz A.I., Bousiquot E., Camosseto V., Samad B., Tsui J., Yan P., et al. 2020. SCENITH: A flow cytometry-based method to functionally profile energy metabolism with single-cell resolution. Cell Metab. 32:1063–1075.e7. 10.1016/j.cmet.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, A., Gordon S.M., Intlekofer A.M., Paley M.A., Mooney E.C., Lindsten T., Wherry E.J., and Reiner S.L.. 2010. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185:4988–4992. 10.4049/jimmunol.1002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentsen, M., Goymann P., Schultheis H., Klee K., Petrova A., Wiegandt R., Fust A., Preussner J., Kuenne C., Braun T., et al. 2020. ATAC-seq footprinting unravels kinetics of transcription factor binding during zygotic genome activation. Nat. Commun. 11:4267. 10.1038/s41467-020-18035-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod, L., Friedrich C., Nandan A., Freitag J., Hagemann S., Harmrolfs K., Sandouk A., Hesse C., Castro C.N., Bähre H., et al. 2014. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20:1327–1333. 10.1038/nm.3704 [DOI] [PubMed] [Google Scholar]
- Bettelli, E., Pagany M., Weiner H.L., Linington C., Sobel R.A., and Kuchroo V.K.. 2003. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197:1073–1081. 10.1084/jem.20021603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, M.D.D., O’Sullivan D., Klein Geltink R.I.I., Curtis J.D.D., Chang C.H., Sanin D.E.E., Qiu J., Kretz O., Braas D., van der Windt G.J.J.W., et al. 2016. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 166:63–76. 10.1016/j.cell.2016.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro, J., Wu B., Chang H., and Greenleaf W.. 2016. ATAC-seq: A method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109:21.29.1–21.29.9. 10.1002/0471142727.mb2129s109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro, J.D., Giresi P.G., Zaba L.C., Chang H.Y., and Greenleaf W.J.. 2013. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods. 10:1213–1218. 10.1038/nmeth.2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne, D.P., Hatle K.M., Fortner K.A., D’Alessandro A., Thornton T.M., Yang R., Torralba D., Tomás-Cortázar J., Jun Y.W., Ahn K.H., et al. 2016. Fine-tuning of CD8(+) T cell mitochondrial metabolism by the respiratory chain repressor MCJ dictates protection to influenza virus. Immunity. 44:1299–1311. 10.1016/j.immuni.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin, K., Ramsköld D., Diaz-Gallo L.M., Herrath J., Houtman M., Tandre K., Rönnblom L., Catrina A., and Malmström V.. 2018. EOMES-positive CD4+ T cells are increased in PTPN22 (1858T) risk allele carriers. Eur. J. Immunol. 48:655–669. 10.1002/eji.201747296 [DOI] [PubMed] [Google Scholar]
- Cogliati, S., Frezza C., Soriano M.E., Varanita T., Quintana-Cabrera R., Corrado M., Cipolat S., Costa V., Casarin A., Gomes L.C., et al. 2013. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 155:160–171. 10.1016/j.cell.2013.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Guilloty, F., Pipkin M.E., Djuretic I.M., Levanon D., Lotem J., Lichtenheld M.G., Groner Y., and Rao A.. 2009. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 206:51–59. 10.1084/jem.20081242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran, M.A., Geiger T.L., Montalvo W., Kim M., Reiner S.L., Al-Shamkhani A., Sun J.C., and Allison J.P.. 2013. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J. Exp. Med. 210:743–755. 10.1084/jem.20121190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, E.V., Barbi J., Yang H.Y., Jinasena D., Yu H., Zheng Y., Bordman Z., Fu J., Kim Y., Yen H.R., et al. 2011. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 146:772–784. 10.1016/j.cell.2011.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daussy, C., Faure F., Mayol K., Viel S., Gasteiger G., Charrier E., Bienvenu J., Henry T., Debien E., Hasan U.A., et al. 2014. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 211:563–577. 10.1084/jem.20131560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean, A.S., Joulia E., and Walzer T.. 2019. The role of Eomes in human CD4 T cell differentiation: A question of context. Eur. J. Immunol. 49:38–41. 10.1002/eji.201848000 [DOI] [PubMed] [Google Scholar]
- Endo, Y., Iwamura C., Kuwahara M., Suzuki A., Sugaya K., Tumes D.J., Tokoyoda K., Hosokawa H., Yamashita M., and Nakayama T.. 2011. Eomesodermin controls interleukin-5 production in memory T helper 2 cells through inhibition of activity of the transcription factor GATA3. Immunity. 35:733–745. 10.1016/j.immuni.2011.08.017 [DOI] [PubMed] [Google Scholar]
- Friedman, J.R., and Nunnari J.. 2014. Mitochondrial form and function. Nature. 505:335–343. 10.1038/nature12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleyzer, N., Vercauteren K., and Scarpulla R.C.. 2005. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell Biol. 25:1354–1366. 10.1128/MCB.25.4.1354-1366.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, L.C., Di Benedetto G., and Scorrano L.. 2011. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13:589–598. 10.1038/ncb2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, S.M., Chaix J., Rupp L.J., Wu J., Madera S., Sun J.C., Lindsten T., and Reiner S.L.. 2012. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 36:55–67. 10.1016/j.immuni.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruarin, P., Maglie S., De Simone M., Häringer B., Vasco C., Ranzani V., Bosotti R., Noddings J.S., Larghi P., Facciotti F., et al. 2019. Eomesodermin controls a unique differentiation program in human IL-10 and IFN-γ coproducing regulatory T cells. Eur. J. Immunol. 49:96–111. 10.1002/eji.201847722 [DOI] [PubMed] [Google Scholar]
- Heinz, S., Benner C., Spann N., Bertolino E., Lin Y.C., Laslo P., Cheng J.X., Murre C., Singh H., and Glass C.K.. 2010. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 38:576–589. 10.1016/j.molcel.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama, K., Sekiya T., Inoue N., Tamiya T., Kashiwagi I., Kimura A., Morita R., Muto G., Shichita T., Takahashi R., and Yoshimura A.. 2011. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-β is mediated by suppression of eomesodermin. Immunity. 34:741–754. 10.1016/j.immuni.2011.02.021 [DOI] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics Consortium . 2019. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 365:eaav7188. 10.1126/science.aav7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer, A.M., Takemoto N., Wherry E.J., Longworth S.A., Northrup J.T., Palanivel V.R., Mullen A.C., Gasink C.R., Kaech S.M., Miller J.D., et al. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6:1236–1244. 10.1038/ni1268 [DOI] [PubMed] [Google Scholar]
- Intlekofer, A.M., Banerjee A., Takemoto N., Gordon S.M., DeJong C.S., Shin H., Hunter C.A., Wherry E.J., Lindsten T., and Reiner S.L.. 2008. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 321:408–411. 10.1126/science.1159806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein Geltink, R.I., O’Sullivan D., Corrado M., Bremser A., Buck M.D., Buescher J.M., Firat E., Zhu X., Niedermann G., Caputa G., et al. 2017. Mitochondrial priming by CD28. Cell. 171:385–397.e11. 10.1016/j.cell.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg, M.D., Bhargava P., Kim P.M., Putluri V., Snowman A.M., Putluri N., Calabresi P.A., and Snyder S.H.. 2018. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science. 360:449–453. 10.1126/science.aan4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy, G., Saxena A., Mars L.T., Domingues H.S., Mentele R., Ben-Nun A., Lassmann H., Dornmair K., Kurschus F.C., Liblau R.S., and Wekerle H.. 2009. Myelin-specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis. Nat. Med. 15:626–632. 10.1038/nm.1975 [DOI] [PubMed] [Google Scholar]
- Krzywinska, E., Sobecki M., Nagarajan S., Zacharjasz J., Tambuwala M.M., Pelletier A., Cummins E., Gotthardt D., Fandrey J., Kerdiles Y.M., et al. 2022. The transcription factor HIF-1α mediates plasticity of NKp46+ innate lymphoid cells in the gut. J. Exp. Med. 219:e20210909. 10.1084/jem.20210909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer, V.A., Tiwari H.K., Reynolds R.J., Danila M.I., Wang J., Edberg J.C., Kimberly R.P., Kottyan L.C., Harley J.B., Mikuls T.R., et al. 2019. Genetic influences on susceptibility to rheumatoid arthritis in African-Americans. Hum. Mol. Genet. 28:858–874. 10.1093/hmg/ddy395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law, P.J., Berndt S.I., Speedy H.E., Camp N.J., Sava G.P., Skibola C.F., Holroyd A., Joseph V., Sunter N.J., Nieters A., et al. 2017. Genome-wide association analysis implicates dysregulation of immunity genes in chronic lymphocytic leukaemia. Nat. Commun. 8:14175. 10.1038/ncomms14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., Zhu B., Son Y.M., Wang Z., Jiang L., Xiang M., Ye Z., Beckermann K.E., Wu Y., Jenkins J.W., et al. 2019. The transcription factor Bhlhe40 programs mitochondrial regulation of resident CD8+ T cell fitness and functionality. Immunity. 51:491–507.e7. 10.1016/j.immuni.2019.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., and 1000 Genome Project Data Processing Subgroup . 2009. The sequence alignment/map format and SAMtools. Bioinformatics. 25:2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.C., Bradstreet T.R., Schwarzkopf E.A., Jarjour N.N., Chou C., Archambault A.S., Sim J., Zinselmeyer B.H., Carrero J.A., Wu G.F., et al. 2016. IL-1-induced Bhlhe40 identifies pathogenic T helper cells in a model of autoimmune neuroinflammation. J. Exp. Med. 213:251–271. 10.1084/jem.20150568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y., Wang Q., Xue G., Bi E., Ma X., Wang A., Qian J., Dong C., and Yi Q.. 2018. Th9 cells represent a unique subset of CD4+ T cells endowed with the ability to eradicate advanced tumors. Cancer Cell. 33:1048–1060.e7. 10.1016/j.ccell.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y., Hong S., Li H., Park J., Hong B., Wang L., Zheng Y., Liu Z., Xu J., He J., et al. 2012. Th9 cells promote antitumor immune responses in vivo. J. Clin. Invest. 122:4160–4171. 10.1172/JCI65459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupar, E., Brack M., Garnier L., Laffont S., Rauch K.S., Schachtrup K., Arnold S.J., Guéry J.-C., and Izcue A.. 2015. Eomesodermin expression in CD4+ T cells restricts peripheral Foxp3 induction. J. Immunol. 195:4742–4752. 10.4049/jimmunol.1501159 [DOI] [PubMed] [Google Scholar]
- Mackay, L.K., Wynne-Jones E., Freestone D., Pellicci D.G., Mielke L.A., Newman D.M., Braun A., Masson F., Kallies A., Belz G.T., and Carbone F.R.. 2015. T-Box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity. 43:1101–1111. 10.1016/j.immuni.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Martínez-Llordella, M., Esensten J.H., Bailey-Bucktrout S.L., Lipsky R.H., Marini A., Chen J., Mughal M., Mattson M.P., Taub D.D., and Bluestone J.A.. 2013. CD28-inducible transcription factor DEC1 is required for efficient autoreactive CD4+ T cell response. J. Exp. Med. 210:1603–1619. 10.1084/jem.20122387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni, A., Maggi L., Siracusa F., Ramazzotti M., Rossi M.C., Santarlasci V., Montaini G., Capone M., Rossettini B., De Palma R., et al. 2019. Eomes controls the development of Th17-derived (non-classic) Th1 cells during chronic inflammation. Eur. J. Immunol. 49:79–95. 10.1002/eji.201847677 [DOI] [PubMed] [Google Scholar]
- McLean, C.Y., Bristor D., Hiller M., Clarke S.L., Schaar B.T., Lowe C.B., Wenger A.M., and Bejerano G.. 2010. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28:495–501. 10.1038/nbt.1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, P., Carelli V., Manfredi G., and Chan D.C.. 2014. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 19:630–641. 10.1016/j.cmet.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley, M.A., Kroy D.C., Odorizzi P.M., Johnnidis J.B., Dolfi D.V., Barnett B.E., Bikoff E.K., Robertson E.J., Lauer G.M., Reiner S.L., et al. 2012. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 338:1220–1225. 10.1126/science.1229620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, E.L., Mullen A.C., Martins G.A., Krawczyk C.M., Hutchins A.S., Zediak V.P., Banica M., DiCioccio C.B., Gross D.A., Mao C.A., et al. 2003. Control of effector CD8+ T cell function by the transcription factor eomesodermin. Science. 302:1041–1043. 10.1126/science.1090148 [DOI] [PubMed] [Google Scholar]
- Pirozyan, M.R., McGuire H.M., Emran A.A., Tseng H.Y., Tiffen J.C., Lee J.H., Carlino M.S., Menzies A.M., Long G.V., Scolyer R.A., et al. 2020. Pretreatment innate cell populations and CD4 T cells in blood are associated with response to immune checkpoint blockade in melanoma patients. Front. Immunol. 11:372. 10.3389/fimmu.2020.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui, H.Z., Hagymasi A.T., Bandyopadhyay S., St Rose M.-C., Ramanarasimhaiah R., Ménoret A., Mittler R.S., Gordon S.M., Reiner S.L., Vella A.T., and Adler A.J.. 2011. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J. Immunol. 187:3555–3564. 10.4049/jimmunol.1101244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan, A.R., and Hall I.M.. 2010. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics. 26:841–842. 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveney, B.J.E., Oki S., Hohjoh H., Nakamura M., Sato W., Murata M., and Yamamura T.. 2015. Eomesodermin-expressing T-helper cells are essential for chronic neuroinflammation. Nat. Commun. 6:8437. 10.1038/ncomms9437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveney, B.J.E., Sato W., Takewaki D., Zhang C., Kanazawa T., Lin Y., Okamoto T., Araki M., Kimura Y., Sato N., et al. 2021. Involvement of cytotoxic Eomes-expressing CD4+ T cells in secondary progressive multiple sclerosis. Proc. Natl. Acad. Sci. USA. 118:e2021818118. 10.1073/pnas.2021818118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner, P.M., Llaó Cid L., Lupar E., Roider T., Bordas M., Schifflers C., Arseni L., Gaupel A.C., Kilpert F., Krötschel M., et al. 2021. EOMES and IL-10 regulate antitumor activity of T regulatory type 1 CD4+ T cells in chronic lymphocytic leukemia. Leukemia. 35:2311–2324. 10.1038/s41375-021-01136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šahmatova, L., Sügis E., Šunina M., Hermann H., Prans E., Pihlap M., Abram K., Rebane A., Peterson H., Peterson P., et al. 2017. Signs of innate immune activation and premature immunosenescence in psoriasis patients. Sci. Rep. 7:7553. 10.1038/s41598-017-07975-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla, R.C. 2008a. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann. N. Y. Acad. Sci. 1147:321–334. 10.1196/annals.1427.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla, R.C. 2008b. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 88:611–638. 10.1152/physrev.00025.2007 [DOI] [PubMed] [Google Scholar]
- Steiner, D.F., Thomas M.F., Hu J.K., Yang Z., Babiarz J.E., Allen C.D.C., Matloubian M., Blelloch R., and Ansel K.M.. 2011. MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity. 35:169–181. 10.1016/j.immuni.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienne, C., Michieletto M.F., Benamar M., Carrié N., Bernard I., Nguyen X.H., Lippi Y., Duguet F., Liblau R.S., Hedrick S.M., et al. 2016. Foxo3 transcription factor drives pathogenic T Helper 1 differentiation by inducing the expression of Eomes. Immunity. 45:774–787. 10.1016/j.immuni.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto, A., Wurster A.L., Reiner S.L., and Grusby M.J.. 2006. IL-21 inhibits IFN-γ production in developing Th1 cells through the repression of Eomesodermin expression. J. Immunol. 177:3721–3727. 10.4049/jimmunol.177.6.3721 [DOI] [PubMed] [Google Scholar]
- Trapnell, C., Pachter L., and Salzberg S.L.. 2009. TopHat: Discovering splice junctions with RNA-seq. Bioinformatics. 25:1105–1111. 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsompana, M., and Buck M.J.. 2014. Chromatin accessibility: A window into the genome. Epigenet. Chromatin. 7:33. 10.1186/1756-8935-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Yu D., Sarnaik A.A., Yu B., Hall M., Morelli D., Zhang Y., Zhao X., and Weber J.S.. 2012. Biomarkers on melanoma patient T cells associated with ipilimumab treatment. J. Transl. Med. 10:146. 10.1186/1479-5876-10-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt, G.J.W., Everts B., Chang C.H., Curtis J.D., Freitas T.C., Amiel E., Pearce E.J., and Pearce E.L.. 2012. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 36:68–78. 10.1016/j.immuni.2011.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Xu J., Niu Y., Bromberg J.S., and Ding Y.. 2008. T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. J. Immunol. 181:8700–8710. 10.4049/jimmunol.181.12.8700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C., Raveney B.J.E., Hohjoh H., Tomi C., Oki S., and Yamamura T.. 2019. Extrapituitary prolactin promotes generation of Eomes-positive helper T cells mediating neuroinflammation. Proc. Natl. Acad. Sci. USA. 116:21131–21139. 10.1073/pnas.1906438116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Le Gras S., Pouxvielh K., Faure F., Fallone L., Kern N., Moreews M., Mathieu A.L., Schneider R., Marliac Q., et al. 2021. Sequential actions of EOMES and T-BET promote stepwise maturation of natural killer cells. Nat. Commun. 12:5446. 10.1038/s41467-021-25758-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., Lee J.S., Gartlan K.H., Schuster I.S., Comerford I., Varelias A., Ullah M.A., Vuckovic S., Koyama M., Kuns R.D., et al. 2017. Eomesodermin promotes the development of type 1 regulatory T (TR1) cells. Sci. Immunol. 2:1–14. 10.1126/sciimmunol.aah7152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X, Koldzic D.N., Izikson L., Reddy J., Nazareno R.F., Sakaguchi S., Kuchroo V.K., and Weiner H.L.. 2004. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int. Immunol. 16:249–256. 10.1093/intimm/dxh029 [DOI] [PubMed] [Google Scholar]
- Zhong, Y., Walker S.K., Pritykin Y., Leslie C.S., Rudensky A.Y., and van der Veeken J.. 2022. Hierarchical regulation of the resting and activated T cell epigenome by major transcription factor families. Nat. Immunol. 23:122–134. 10.1038/s41590-021-01086-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., Ju S., Chen E., Dai S., Li C., Morel P., Liu L., Zhang X., and Lu B.. 2010. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J. Immunol. 185:3174–3183. 10.4049/jimmunol.1000749 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lists the differential accessible regions comparing Eomes-GFP+ and Eomes-TKO CD4+ T cells upon in vitro stimulation by ATAC-seq.
maps the genomic occupancy of Eomes in CD4+ T cells by CUT&RUN assay comparing stimulated CD4+ T cells from Eomes-GFP+ and Eomes-TKO mice.
lists the regions of Eomes footprints at Eomes-GFP+ open regions (ATAC) overlapping Eomes binding sites (CUT&RUN) using the TOBIAS tool.
lists antibodies, clones, fluorochromes, reagents used with suppliers.
Data Availability Statement
The data underlying Figs. 1, 2, 3, 6, and 7 are available in the published article and its online supplemental material. The data underlying Figs. 4 and 5 related to RNA-seq, ATAC-seq, and CUT&RUN datasets are openly available at the Gene Expression Omnibus under accession no. GSE247863. The following published datasets were used in addition: accession nos. GSE135278 (Li et al., 2019) and GSE166718 (Zhong et al., 2022).