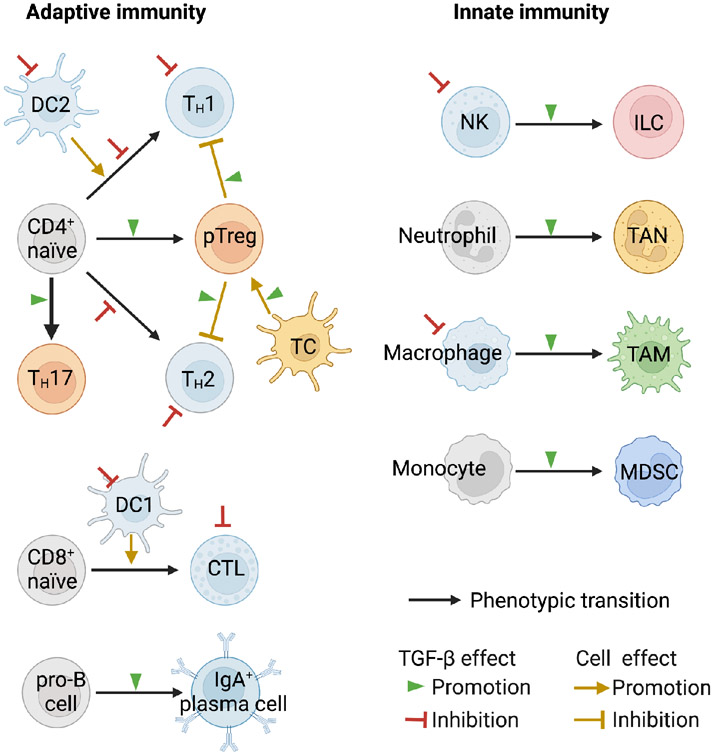
Figure 3. TGF-β and immune regulation.
Scheme of the main classes of immune cells and their regulation by TGF-β in the adult. TGF-β is a critical modulator of both adaptive and innate immunity arms, acting as a general enforcer of immune tolerance and a suppressor of inflammation. In the adaptive arm, TGF-β inhibits the maturation of naïve CD4+ T cells into TH1 and TH2 T helper cells and of naïve CD8+ T cell into cytotoxic T lymphocytes (CTL). TGF-β exerts these effects through direct inhibition of CD4+ and CD8+ maturation and through inhibition of dendritic cell subsets (DC1, DC2) that drive naïve these maturation steps. TGF-β additionally inhibits the helper functions of TH1 and TH2, and the effector functions of CTL cells, and it can do so by acting directly on these cells as well as by promoting the differentiation of CD4+ T cells into peripheral regulatory T cells (pTreg), which inhibit TH1 and TH2 cells partly through TGF-β. A specialized RORγt+ antigen-presenting cell (TC ) activates pTreg cells in the intestinal lymph nodes. TGF-β inhibits B cell proliferation but stimulates IgA class switching in B cells. In the innate immunity arm, TGF-β blunts the effector functions of natural killer (NK) cells, and the inflammatory functions of neutrophils and macrophages while favoring, in the context of tumors, the adoption of tumor-associated neutrophil (TAN) and macrophage (TAM) states which support tumor progression. In chronic infection, inflammation, and cancer, the persistent myelopoiesis includes production of myeloid-derived suppressor cells (MDSC) with TGF-β dependent immunosuppressive functions. These regulatory effects of TGF-β on the immune system occur to different extents in different tissue contexts and depending on whether the circumstance is homeostasis, acute injury or infection, or chronic inflammation, fibrosis, or cancer.