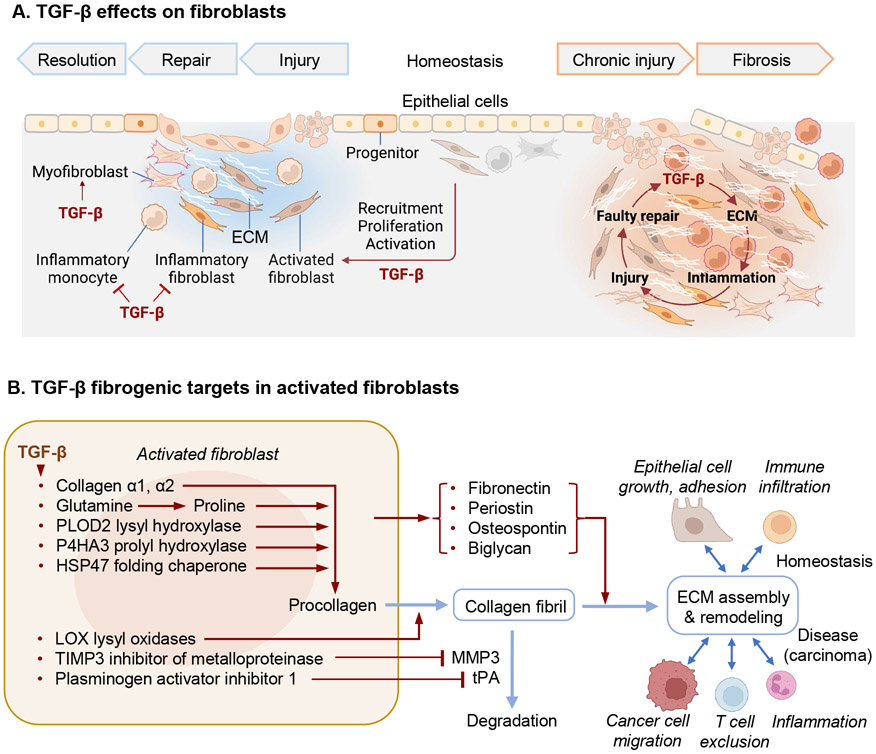
Figure 4. TGF-β regulation of fibroblasts in health and disease.
A. Main effects of TGF-β on fibroblasts during injury repair and chronic fibrosis, and impact on epithelial and immune cells. TGF-β regulates fibroblast activity throughout the tissue response to injury and the return to homeostasis (left side) as well as during chronic fibrosis (right side). TGF-β potently induces the recruitment, proliferation and activation of fibroblast that produce collagens, fibronectin, and other components required for ECM assembly, as well as integrins that mediate cell adhesion to the ECM. Activated fibroblasts additionally establish paracrine communication with epithelial cells, angiogenic progenitors, and local innate and adaptive immune functions. TGF-β also induces a highly contractile myofibroblast phenotype expressing α-smooth muscle actin. These phenotypes appear to emerge at the expense of a pro-inflammatory fibroblast phenotype, while TGF-β additionally restricts inflammatory monocytes. ECM deposition and remodeling is essential for epithelial progenitors to reconstitute the barrier tissue after injury. Tissue fibrosis, characterized by chronic inflammation and accumulation of fibrillar collagens and other ECM components resulting from imbalanced production of ECM by tissue resident fibroblasts. Feed-forward loops involving TGF-β contribute to fibrosis by exaggerating normal physiologic responses and triggering further epithelial injury and inflammation. B. TGF-β potently induces expression of fibrillar collagens as well as the metabolic adaptations, enzymes, and chaperones required for the biosynthesis and ECM deposition of collagen fibrils. TGF-β induces expression of additional ECM components in fibroblasts and epithelial cells. The production and turnover of ECM is a complex process requiring inputs from epithelial cells, innate and adaptive immune cells, and other cell types. Intratumoral fibrosis contributes to the exclusion of T cells from tumors. PLOD2, procollagen-lysine,2-oxoglutarate 5-deoxygenase 2; P4HA3, prolyl-4-hydroxylase 3, catalyzes proline hydroxylation; HSP47, heat-shock protein 47; LOX, lysyl oxidase; TIMP3, tissue inhibitor of metalloproteinase 3.