Abstract
The use of peripheral blood (PB) or bone marrow (BM) stem cells graft in haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide (PTCy) for graft-versus-host disease (GVHD) prophylaxis remains controversial. Moreover, the value of adding anti-thymoglobulin (ATG) to PTCy is unknown. A total of 1344 adult patients received an unmanipulated haploidentical transplant at 37 centers from 2012 to 2019 for hematologic malignancy. We compared the outcomes of patients according to the type of graft, using a propensity score analysis. In total population, grade II–IV and III–IV acute GVHD (aGVHD) were lower with BM than with PB. Grade III–IV aGVHD was lower with BM than with PB + ATG. All outcomes were similar in PB and PB + ATG groups. Then, in total population, adding ATG does not benefit the procedure. In acute leukemia, myelodysplastic syndrome and myeloproliferative syndrome (AL-MDS-MPS) subgroup receiving non-myeloablative conditioning, risk of relapse was twice greater with BM than with PB (51 vs. 22%, respectively). Conversely, risk of aGVHD was greater with PB (38% for aGVHD II–IV; 16% for aGVHD III–IV) than with BM (28% for aGVHD II–IV; 8% for aGVHD III–IV). In this subgroup with intensified conditioning regimen, risk of relapse became similar with PB and BM but risk of aGVHD III–IV remained higher with PB than with BM graft (HR = 2.0; range [1.17–3.43], p = 0.012).
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-023-01515-4.
Keywords: Haploidentical hematopoietic stem cell transplantation, Graft source, Bone marrow, Peripheral stem cell, Anti-thymoglobulin
To the Editor,
Haploidentical hematopoietic stem cell transplantations account for a quarter of allogeneic HSCT worldwide [1–3]. There is no consensus on the use of bone marrow (BM) versus peripheral blood (PB) stem cells and on the value of adding anti-thymoglobulin (ATG) to post-transplant cyclophosphamide (PTCy) [4–7]. We report the experience of the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) retrospectively comparing the use of BM versus PB versus PB + ATG in patients who received T cell repleted graft from a haploidentical familial donor with PTCy between 2012 and 2019 in 37 centers. In addition, subgroup analyses of acute leukemia, myelodysplastic syndrome and myeloproliferative syndrome (AL-MDS-MPS) were performed according to conditioning intensity. A propensity score was used to make these different groups comparable (Additional files 1, 4).
Findings
A total of 1344 patients were reported, including 371 BM, 776 PB without ATG and 197 received PB with ATG. The median age at transplant was 56 (IQR, 40.7–63.7). Patients were treated for AL (56%) or another myeloid (24%) or lymphoid (20%) malignancies. Myeloablative conditioning (MAC) regimen was more frequently used with BM (p < 0.05). PB + ATG patients had higher disease risk index score than other patients (p < 0.0001). Median follow-up was 28.7 months (Additional file 3: Table S1).
In the total population, engraftment and platelet reconstitution were similar between the three groups (Additional file 3: Table S2).
The cumulative incidence (CI) of 3-month aGVHD II–IV and aGVHD III–IV was 27.9%, 38.3%, 34.2%, and 7.7%, 15.9%,17.3% with BM, PB and PB + ATG, respectively (p < 0.05). The CI of two-year extensive chronic GVHD (cGVHD) was 11.8% without difference in the groups. After adjustments, the risk of aGVHD was lower with BM than with PB grafts. The risk of aGVHD III–IV was lower with BM than with PB + ATG and the risk of TRM was similar between the groups. The CI of relapse at two years was 28%, 25% and 30% with BM, PB and PB + ATG, respectively (p = 0.31), and there was no difference after weighting. The probability of two-year overall survival (OS) was 60.3%, 54.1% and 42.4% with BM, PB and PB + ATG, respectively (p < 0.05), but there was no difference after adjustments. Then, after adjustments, the risk of two-year GVHD-and-relapse-free-survival (GRFS) remained lower with BM than with PB + ATG (Additional file 3: Table S3).
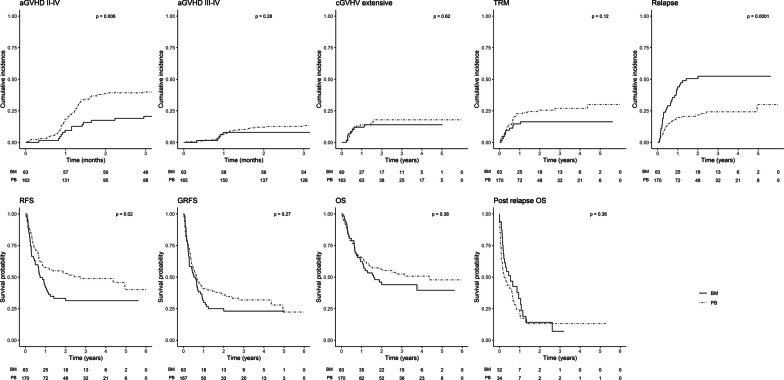
Analyses then focused on a subgroup of diseases at high risk of relapse, AL-MDS-MPS, and excluding patients receiving ATG. Conditioning regimens were grouped into two categories: NMA Baltimore-type and more intensive regimens (MAC and reduced intensity conditioning (RIC) excluding NMA). In the setting of NMA regimen and after adjustments, there was a twofold increased risk of aGVHD II–IV as well as a twofold lower risk of relapse with PB than with BM grafts (Fig. 1; Additional file 3: Table S4).
Fig. 1.
Cumulative incidence of outcomes for AL-MDS-MPS with NMA conditioning, according to graft
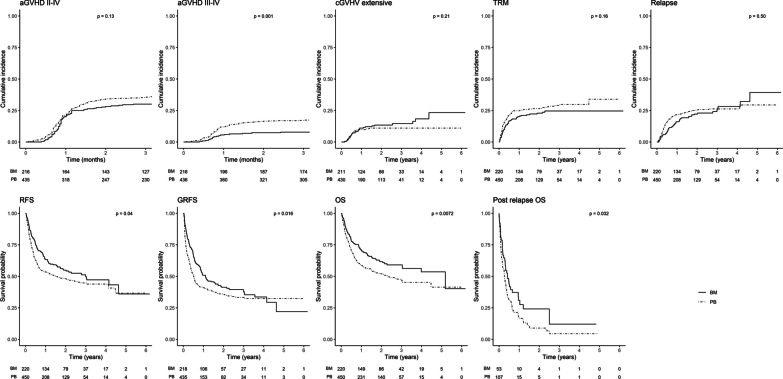
With more intensive conditioning regimens, the risk of aGVHD III–IV remained twice higher with PB without any difference in risk of others outcomes of interests, in particular without impact on relapse (Fig. 2; Additional file 3: Table S5).
Fig. 2.
Cumulative incidence of outcomes for AL-MDS-MPS with intensive conditioning, according to graft
Discussion
In line with previous studies reporting aGVHD rates of 25% for transplants with BM grafts versus 40% with PB grafts, we found that PB graft was associated with higher rates of aGVHD compared to BM [4–10]. Adding ATG did not decrease the rate of GVHD. This is in contradiction with previous studies reporting a decrease in the rate of cGVHD when ATG was added to PTCy [11]. However, these studies were based on a limited number of patients, had included various doses of ATG or Cy-PT, and reported various grades of cGVHD. We report that in the subgroup of patients with AL-MDS-MPS, the risk of relapse is greatly increased with BM associated with NMA, whereas this risk is no longer observed when conditioning is intensified. Other studies have reported an increased risk of relapse in patients with leukemia after BM graft, or a reduction in relapses after MAC [5, 12]. However, no study has performed a combined analysis of relapse risk in leukemia patients according to graft type and conditioning intensity, possibly due to insufficient population size. This is the largest series of haploidentical transplants with PTCy reported to date. Although retrospective, statistical analysis using the propensity score makes it possible to homogenize the sub-populations and make them comparable. The large number of patients studied makes it possible to analyze the impact of the “intensity of conditioning regimen” factor in addition to graft type and disease type. We feel it is important to remember that graft outcome depends on many factors, and that these three elements must be considered to optimize the hematological treatment (Additional file 2).
Supplementary Information
Additional file 1. Supplementary materials and methods.
Additional file 2. Supplementary discussion.
Additional file 3. Supplementary tables.
Additional file 4. Supplementary figures.
Acknowledgements
We acknowledge all the patients included in the study. We also acknowledge the SFGM-TC for patient recruitment, particularly Nicole Raus, data manager, for helping the authors during data collection.
Abbreviations
- ATG
Anti-thymoglobulin
- BM
Bone marrow
- CI
Cumulative incidence
- GVHD
Graft-versus-host disease
- aGVHD
Acute graft-versus-host disease
- cGVHD
Chronic graft-versus-host disease
- GRFS
GVHD-free/relapse-free survival
- HR
Hazard ratio
- IQR
Interquartile range
- MAC
Myeloablative conditioning
- NMA
Non-myeloablative
- OS
Overall survival
- PB
Peripheral blood
- PTCy
Post-transplant cyclophosphamide
- RIC
Reduced intensity conditioning
- TRM
Treatment-related mortality
Author contributions
CL was principal investigator. SN and RD were senior authors and contributed equally to co-last author. CL, SN, JL and AWP analyzed and interpreted data, wrote the manuscript and created the table and figure; CL collected data; CL, EF, MR, PC, SL, CEB, CO, PC, ED, AC, YC, MB, CS, MTR, PT, JM, AH, ML, JOB, GG, MA, CCL, XP, SC, NM, YB, AM, JC, JVM, SM, NM, AV, MAB, NJ, MS, RD and SN provided patient care. All the authors agreed to the final version of the manuscript.
Funding
Not applicable.
Availability of data and materials
All clinical and biological data have been shared within the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) Promise database. External users with a formal analysis plan may request access to these data from corresponding author.
Declarations
Ethics approval and consent to participate
The Institutional Review Board of the SFGM-TC approved the study.
Consent for publication
All patients provided written informed consent for their data to be used for clinical research in accordance with the modified Declaration of Helsinki and Good Clinical Practice guidelines.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazarbachi A, Boumendil A, Finel H, Castagna L, Dominietto A, Blaise D, et al. Influence of donor type, stem cell source and conditioning on outcomes after haploidentical transplant for lymphoma—a LWP-EBMT study. Br J Haematol. 2020;188(5):745–756. doi: 10.1111/bjh.16182. [DOI] [PubMed] [Google Scholar]
- 4.Castagna L, Bramanti S, Furst S, Giordano L, Crocchiolo R, Sarina B, et al. Nonmyeloablative conditioning, unmanipulated haploidentical SCT and post-infusion CY for advanced lymphomas. Bone Marrow Transplant. 2014;49(12):1475–1480. doi: 10.1038/bmt.2014.197. [DOI] [PubMed] [Google Scholar]
- 5.Bashey A, Zhang MJ, McCurdy SR, St. Martin A, Argall T, Anasetti C, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell–replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35(26):3002–3009. doi: 10.1200/JCO.2017.72.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggeri A, Labopin M, Bacigalupo A, Gülbas Z, Koc Y, Blaise D, et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer. 2018;124(7):1428–1437. doi: 10.1002/cncr.31228. [DOI] [PubMed] [Google Scholar]
- 7.Mussetti A, De Philippis C, Carniti C, Bastos-Oreiro M, Gayoso J, Cieri N, et al. CD3+ graft cell count influence on chronic GVHD in haploidentical allogeneic transplantation using post-transplant cyclophosphamide. Bone Marrow Transplant. 2018;53(12):1522–1531. doi: 10.1038/s41409-018-0183-8. [DOI] [PubMed] [Google Scholar]
- 8.Bashey A, Zhang X, Jackson K, Brown S, Ridgeway M, Solh M, et al. Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: a multivariable analysis including disease risk index. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2016;22(1):125–133. doi: 10.1016/j.bbmt.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell PV, Eapen M, Horowitz MM, Logan BR, DiGilio A, Brunstein C, et al. Comparable outcomes with marrow or peripheral blood as stem cell sources for hematopoietic cell transplantation from haploidentical donors after non-ablative conditioning: a matched-pair analysis. Bone Marrow Transplant. 2016;51(12):1599–1601. doi: 10.1038/bmt.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagler A, Dholaria B, Labopin M, Savani BN, Angelucci E, Koc Y, et al. Bone marrow versus mobilized peripheral blood stem cell graft in T-cell-replete haploidentical transplantation in acute lymphoblastic leukemia. Leukemia. 2020;34(10):2766–2775. doi: 10.1038/s41375-020-0850-9. [DOI] [PubMed] [Google Scholar]
- 11.Duléry R, Brissot E, Mohty M. Combining post-transplant cyclophosphamide with antithymocyte globulin for graft-versus-host disease prophylaxis in hematological malignancies. Blood Rev. 2023 doi: 10.1016/j.blre.2023.101080. [DOI] [PubMed] [Google Scholar]
- 12.Arcuri LJ, Hamerschlak N, Rocha V, Bonfim C, Kerbauy MN. Outcomes after haploidentical hematopoietic cell transplantation with post-transplantation cyclophosphamide: a systematic review and meta-analysis comparing myeloablative with reduced-intensity conditioning regimens and bone marrow with peripheral blood stem cell grafts. Transplant Cell Ther. 2021;27(9):782.e1–782.e7. doi: 10.1016/j.jtct.2021.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary materials and methods.
Additional file 2. Supplementary discussion.
Additional file 3. Supplementary tables.
Additional file 4. Supplementary figures.
Data Availability Statement
All clinical and biological data have been shared within the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) Promise database. External users with a formal analysis plan may request access to these data from corresponding author.