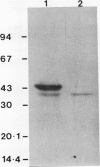
Abstract
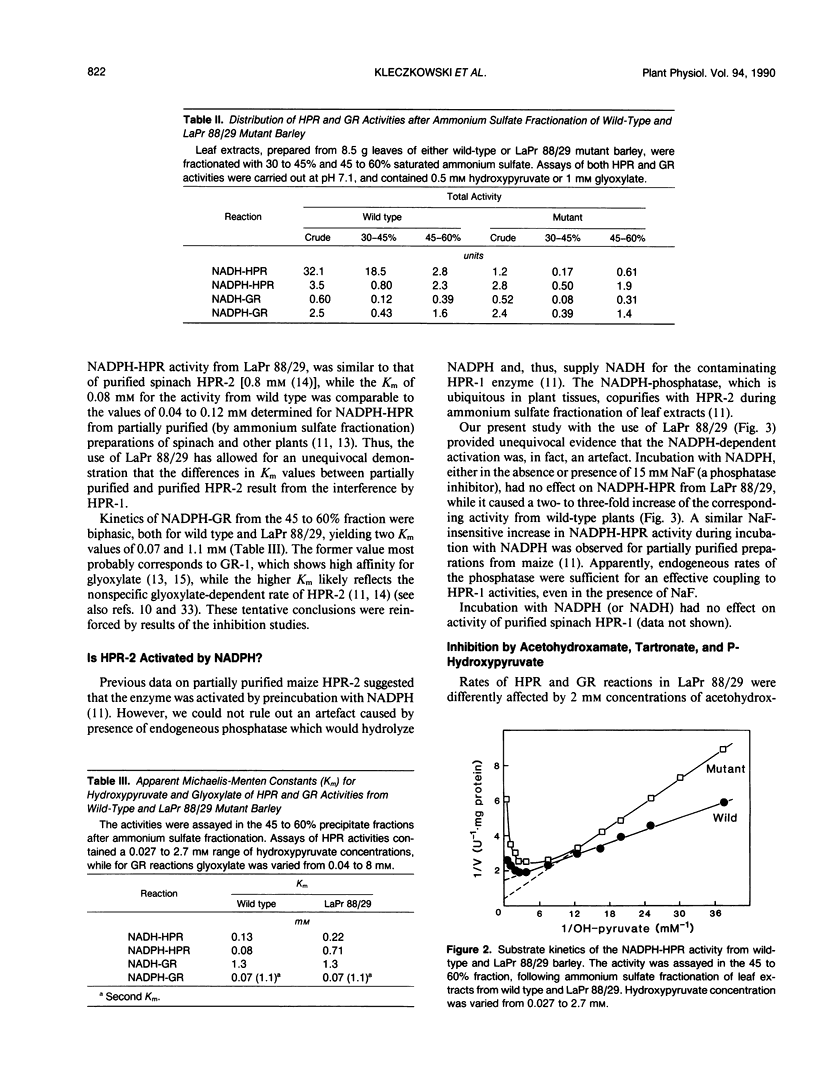
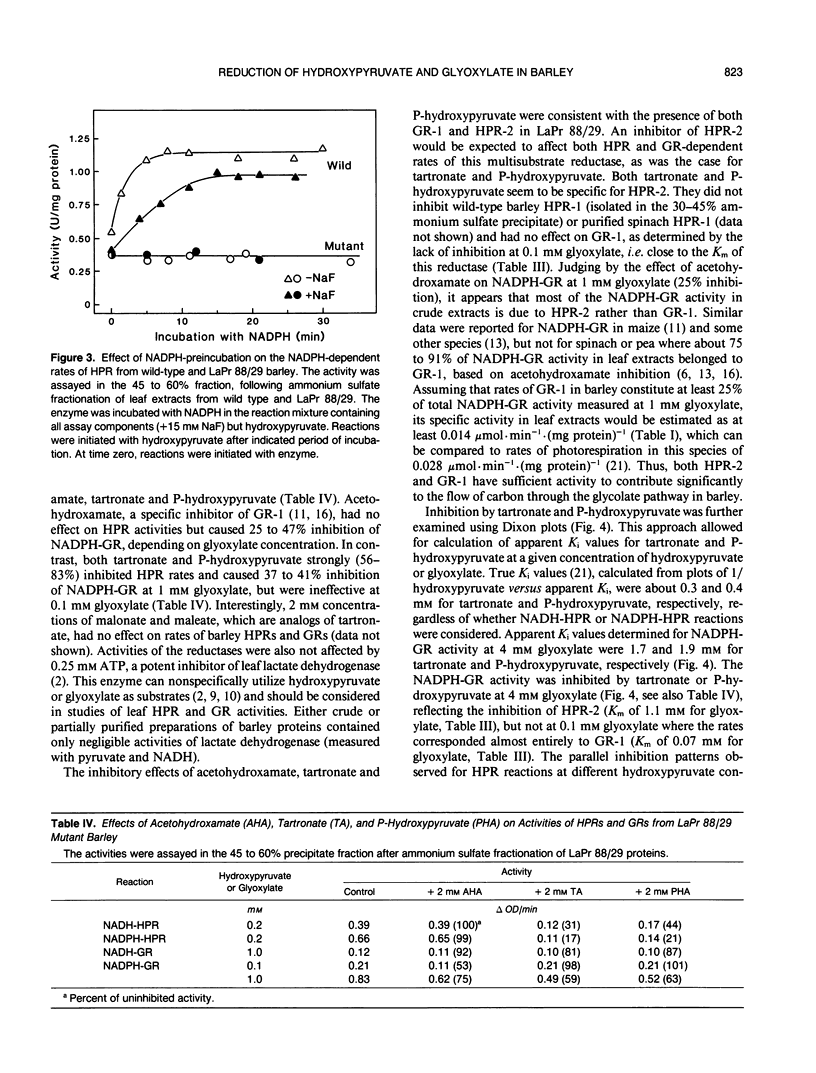
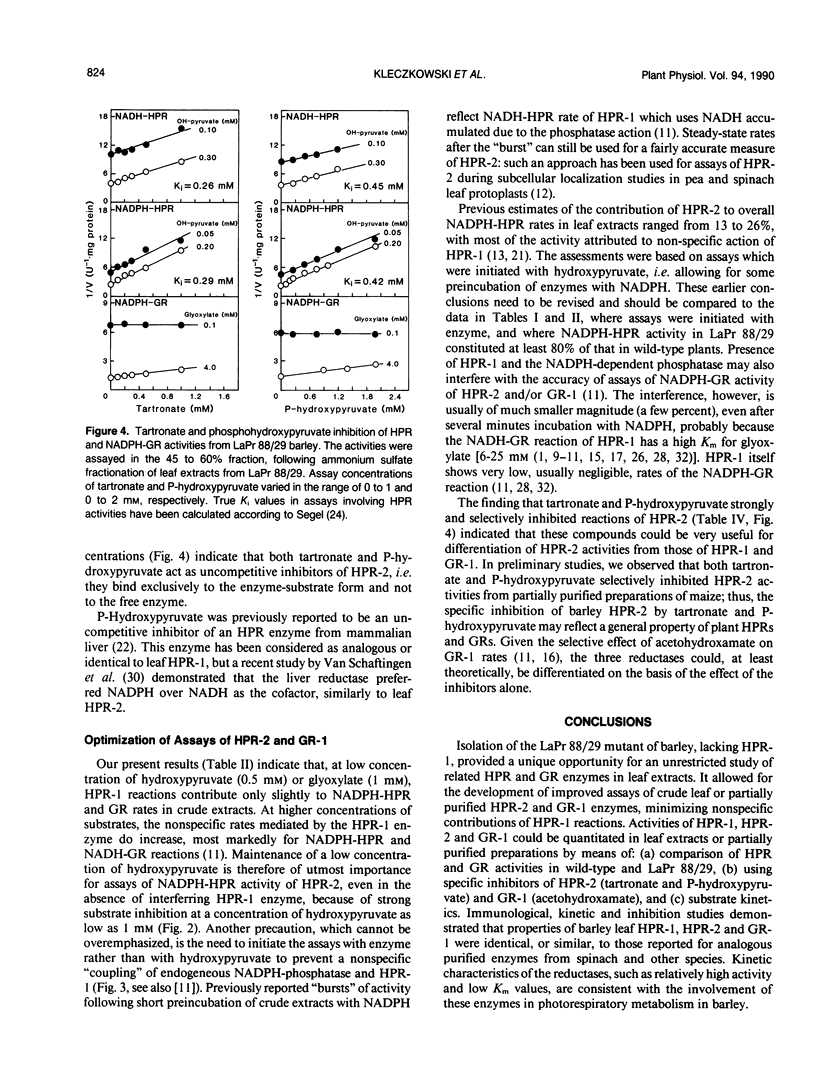
The use of LaPr 88/29 mutant of barley (Hordeum vulgare), which lacks NADH-preferring hydroxypyruvate reductase (HPR-1), allowed for an unequivocal demonstration of at least two related NADPH-preferring reductases in this species: HPR-2, reactive with both hydroxypyruvate and glyoxylate, and the glyoxylate specific reductase (GR-1). Antibodies against spinach HPR-1 recognized barley HPR-1 and partially reacted with barley HPR-2, but not GR-1, as demonstrated by Western immunoblotting and immunoprecipitation of proteins from crude leaf extracts. The mutant was deficient in HPR-1 protein. In partially purified preparations, the activities of HPR-1, HPR-2, and GR-1 could be differentiated by substrate kinetics and/or inhibition studies. Apparent Km values of HPR-2 for hydroxypyruvate and glyoxylate were 0.7 and 1.1 millimolar, respectively, while the Km of GR-1 for glyoxylate was 0.07 millimolar. The Km values of HPR-1, measured in wild type, for hydroxypyruvate and glyoxylate were 0.12 and 20 millimolar, respectively. Tartronate and P-hydroxypyruvate acted as selective uncompetitive inhibitors of HPR-2 (Ki values of 0.3 and 0.4 millimolar, respectively), while acetohydroxamate selectively inhibited GR-1 activity. Nonspecific contributions of HPR-1 reactions in assays of HPR-2 and GR-1 activities were quantified by a direct comparison of rates in preparations from wild-type and LaPr 88/29 plants. The data are evaluated with respect to previous reports on plant HPR and GR activities and with respect to optimal assay procedures for individual HPR-1, HPR-2, and GR-1 rates in leaf preparations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betsche T. L-Lactate dehydrogenase from leaves of higher plants. Kinetics and regulation of the enzyme from lettuce (Lactuca sativa L). Biochem J. 1981 Jun 1;195(3):615–622. doi: 10.1042/bj1950615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Grant G. A. A new family of 2-hydroxyacid dehydrogenases. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1371–1374. doi: 10.1016/0006-291x(89)92755-1. [DOI] [PubMed] [Google Scholar]
- Greenler J. M., Sloan J. S., Schwartz B. W., Becker W. M. Isolation, characterization and sequence analysis of a full-length cDNA clone encoding NADH-dependent hydroxypyruvate reductase from cucumber. Plant Mol Biol. 1989 Aug;13(2):139–150. doi: 10.1007/BF00016133. [DOI] [PubMed] [Google Scholar]
- Husic D. W., Tolbert N. E. NADH:hydroxypyruvate reductase and NADPH:glyoxylate reductase in algae: partial purification and characterization from Chlamydomonas reinhardtii. Arch Biochem Biophys. 1987 Feb 1;252(2):396–408. doi: 10.1016/0003-9861(87)90046-4. [DOI] [PubMed] [Google Scholar]
- Kleczkowski L. A., Edwards G. E. Identification of hydroxypyruvate and glyoxylate reductases in maize leaves. Plant Physiol. 1989 Sep;91(1):278–286. doi: 10.1104/pp.91.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Givan C. V., Hodgson J. M., Randall D. D. Subcellular Location of NADPH-Dependent Hydroxypyruvate Reductase Activity in Leaf Protoplasts of Pisum sativum L. and Its Role in Photorespiratory Metabolism. Plant Physiol. 1988 Dec;88(4):1182–1185. doi: 10.1104/pp.88.4.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D., Blevins D. G. Inhibition of Spinach Leaf NADPH(NADH)-Glyoxylate Reductase by Acetohydroxamate, Aminooxyacetate, and Glycidate. Plant Physiol. 1987 Jul;84(3):619–623. doi: 10.1104/pp.84.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D., Blevins D. G. Purification and characterization of a novel NADPH(NADH)-dependent glyoxylate reductase from spinach leaves. Comparison of immunological properties of leaf glyoxylate reductase and hydroxypyruvate reductase. Biochem J. 1986 Nov 1;239(3):653–659. doi: 10.1042/bj2390653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D. Purification and characterization of a novel NADPH(NADH)-dependent hydroxypyruvate reductase from spinach leaves. Comparison of immunological properties of leaf hydroxypyruvate reductases. Biochem J. 1988 Feb 15;250(1):145–152. doi: 10.1042/bj2500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L. D., Warren W. A. The kinetic properties of spinach leaf glyoxylic acid reductase. J Biol Chem. 1970 Aug 10;245(15):3831–3839. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murray A. J., Blackwell R. D., Lea P. J. Metabolism of Hydroxypyruvate in a Mutant of Barley Lacking NADH-Dependent Hydroxypyruvate Reductase, an Important Photorespiratory Enzyme Activity. Plant Physiol. 1989 Sep;91(1):395–400. doi: 10.1104/pp.91.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum I. Y., Antkowiak D. H., Sallach H. J., Flanders L. E., Fahien L. A. Purification and regulatory properties of beef liver D-glycerate dehydrogenase. Arch Biochem Biophys. 1971 May;144(1):375–383. doi: 10.1016/0003-9861(71)90490-5. [DOI] [PubMed] [Google Scholar]
- Schmitt M. R., Edwards G. E. Provisions of reductant for the hydroxypyruvate to glycerate conversion in leaf peroxisomes : a critical evaluation of the proposed malate/aspartate shuttle. Plant Physiol. 1983 Jul;72(3):728–734. doi: 10.1104/pp.72.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus D. E., Hondred D., Becker W. M. Purification and characterization of hydroxypyruvate reductase from cucumber cotyledons. Plant Physiol. 1983 Jun;72(2):402–408. doi: 10.1104/pp.72.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey K. L., Grant G. A. The nucleotide sequence of the serA gene of Escherichia coli and the amino acid sequence of the encoded protein, D-3-phosphoglycerate dehydrogenase. J Biol Chem. 1986 Sep 15;261(26):12179–12183. [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Draye J. P., Van Hoof F. Coenzyme specificity of mammalian liver D-glycerate dehydrogenase. Eur J Biochem. 1989 Dec 8;186(1-2):355–359. doi: 10.1111/j.1432-1033.1989.tb15216.x. [DOI] [PubMed] [Google Scholar]
- Yu C., Huang A. H. Conversion of serine to glycerate in intact spinach leaf peroxisomes: role of malate dehydrogenase. Arch Biochem Biophys. 1986 Feb 15;245(1):125–133. doi: 10.1016/0003-9861(86)90196-7. [DOI] [PubMed] [Google Scholar]
- ZELITCH I., GOTTO A. M. Properties of a new glyoxylate reductase from leaves. Biochem J. 1962 Sep;84:541–546. doi: 10.1042/bj0840541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I. The isolation and action of crystalline glyoxylic acid reductase from tobacco leaves. J Biol Chem. 1955 Oct;216(2):553–575. [PubMed] [Google Scholar]