Abstract
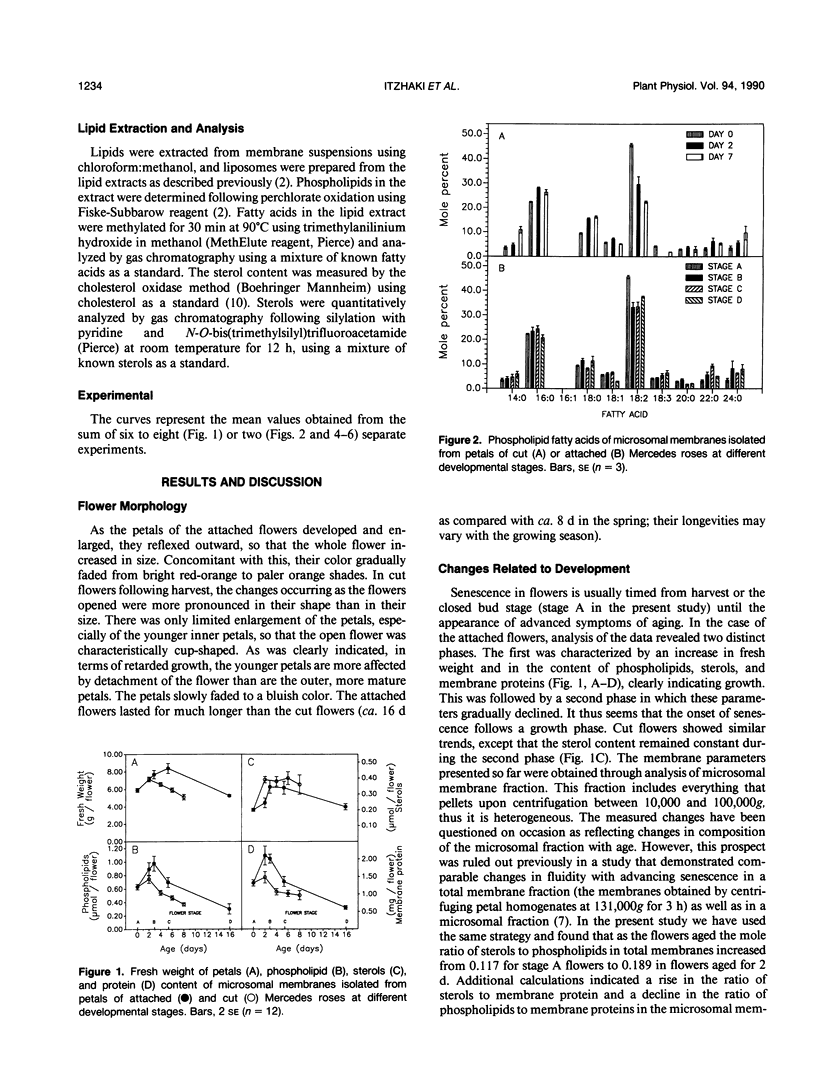
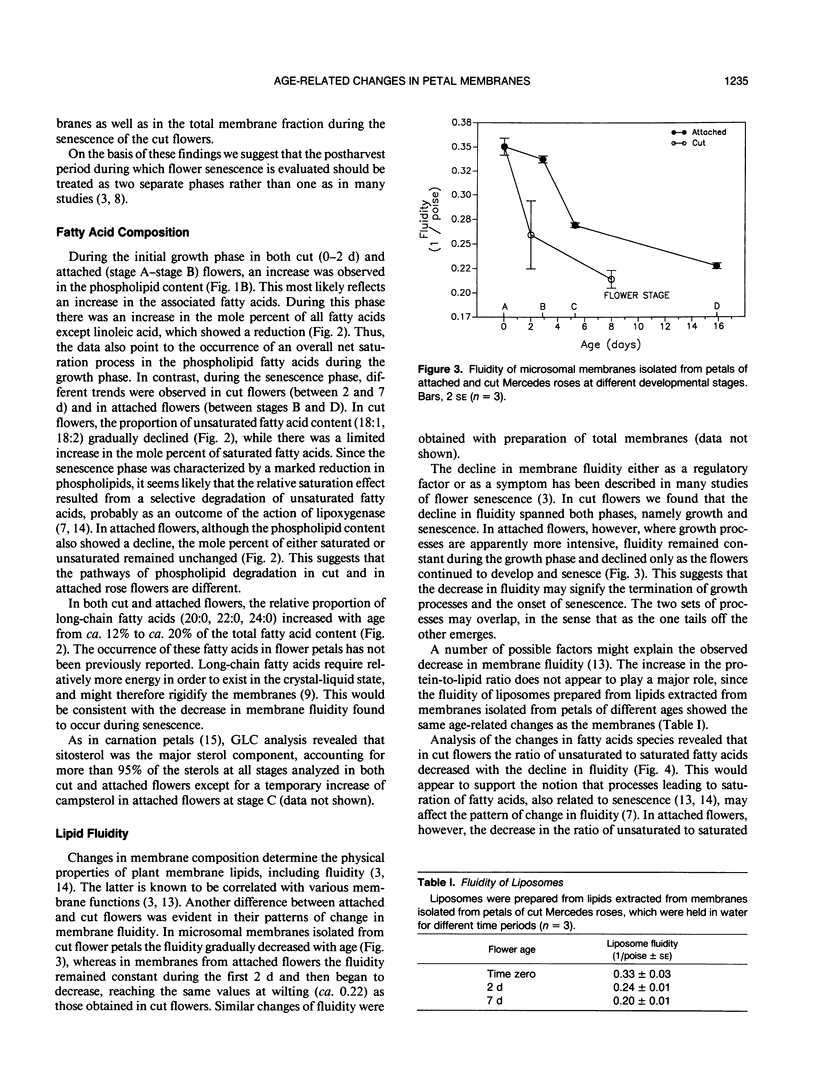
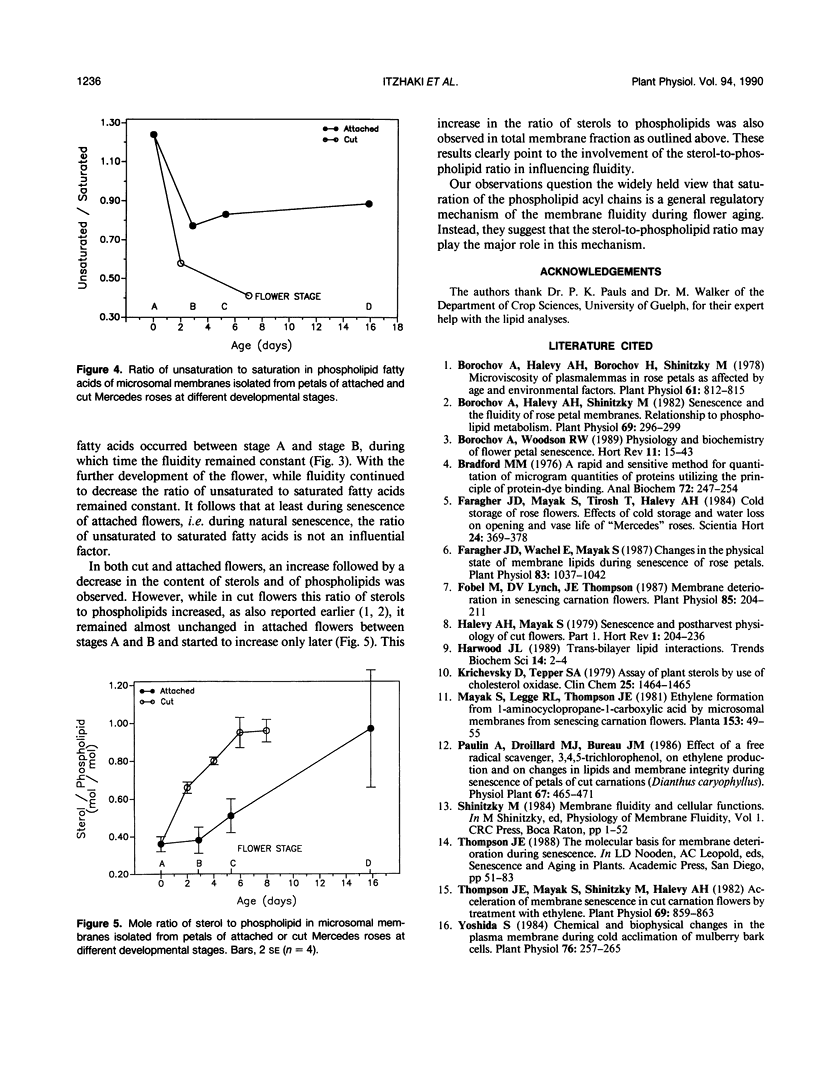
Changes in petal membrane properties during aging were studied in cut and in attached rose flowers (Rosa hybrida L., cv Mercedes). Both cut and attached flowers exhibited a growth phase characterized by an increase in fresh weight and an accumulation of membrane components. The growth phase, which was more pronounced in the attached than in the cut flowers, was followed by a senescence phase, characterized by a decrease in fresh weight and a decline in membrane components. In cut flowers, both the growth and the senescence phases were accompanied by a decrease in membrane fluidity and in the ratio of unsaturated to saturated fatty acids, but the ratio of sterol to phospholipid increased. In attached flowers, while both the membrane fluidity and the sterol-to-phospholipid ratio remained unchanged during the growth phase, the senescence phase was accompanied (as in cut flowers) by a decrease in membrane fluidity and an increase in the sterol-to-phospholipid ratio. Unlike in cut flowers, however, the age-related changes in the ratio of unsaturation of fatty acids were not correlated with those of fluidity. Changes in the saturation of phospholipid acyl chains are commonly thought to influence membrane fluidity. Our observations question this view and suggest instead that the ratio of sterol to phospholipid may play the major role in maintaining membrane lipid fluidity.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borochov A., Halevy A. H. Microviscosity of plasmalemmas in rose petals as affected by age and environmental factors. Plant Physiol. 1978 May;61(5):812–815. doi: 10.1104/pp.61.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov A., Halevy A. H., Shinitzky M. Senescence and the Fluidity of Rose Petal Membranes : RELATIONSHIP TO PHOSPHOLIPID METABOLISM. Plant Physiol. 1982 Feb;69(2):296–299. doi: 10.1104/pp.69.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Faragher J. D., Wachtel E., Mayak S. Changes in the Physical State of Membrane Lipids during Senescence of Rose Petals. Plant Physiol. 1987 Apr;83(4):1037–1042. doi: 10.1104/pp.83.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fobel M., Lynch D. V., Thompson J. E. Membrane deterioration in senescing carnation flowers : coordinated effects of phospholipid degradation and the action of membranous lipoxygenase. Plant Physiol. 1987 Sep;85(1):204–211. doi: 10.1104/pp.85.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky D., Tepper S. A. Assay of plant sterols by use of cholesterol oxidase. Clin Chem. 1979 Aug;25(8):1464–1465. [PubMed] [Google Scholar]
- Thompson J. E., Mayak S., Shinitzky M., Halevy A. H. Acceleration of membrane senescence in cut carnation flowers by treatment with ethylene. Plant Physiol. 1982 Apr;69(4):859–863. doi: 10.1104/pp.69.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Chemical and Biophysical Changes in the Plasma Membrane during Cold Acclimation of Mulberry Bark Cells (Morus bombycis Koidz. cv Goroji). Plant Physiol. 1984 Sep;76(1):257–265. doi: 10.1104/pp.76.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]


