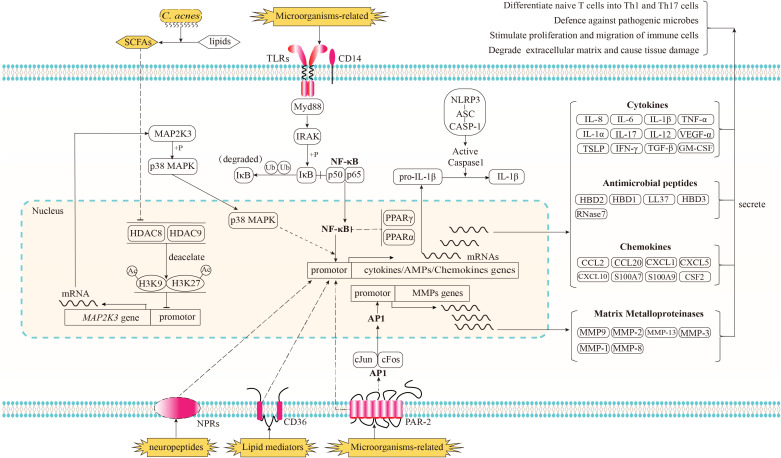
Figure 1.
Recognition of immune stimulators and the signaling pathways implicated in the innate immune response of acne and its subsequent biological effects. Microorganisms-related stimulators can be detected by Toll-like receptors (TLRs) in conjunction with CD14 and proteinase-activated receptor-2 (PAR2). The activation of TLRs triggers the downstream NF-κB signaling pathway, resulting in the translocation of NF-κB into the nucleus and the upregulation of genes encoding cytokines, chemokines, and antimicrobial peptides (AMPs); The activation of PAR2 has been shown to elicit the transcriptional upregulation of genes encoding cytokines, chemokines, and AMPs via an unidentified pathway. Additionally, PAR2 activation triggers the downstream signaling pathway of activator protein-1 (AP-1), resulting in the translocation of AP-1 into the nucleus and an enhanced transcriptional expression of matrix metalloproteinases (MMPs). As potent anti-inflammatory factors, the nuclear receptors, peroxisome proliferator-activated receptors (PPARs) PPARα and PPARγ have the ability to inhibit the activation of NF-κB. When cultivated in an environment rich in lipids, the anaerobic fermentation of C. acnes can produce short chain free fatty acids (SCFAs). Certain species of SCFAs have the ability to inhibit the deacetylation function of histone deacetylase (HDAC) 8/9. The inhibition of HDAC8/9 consequently results in an amplification of the acetylation process on histone residues H3K9 and H3K27, which marker the promoter region of MAP2K3. This, in turn, leads to an enhanced transcription of MAP2K3. The heightened expression level of MAP2K3 then triggers the phosphorylation of p38 MAPK, ultimately resulting in the activation of p38 MAPK and an increase in the expression of genes responsible for cytokines and chemokines. Lipid mediators produced by sebaceous glands, such as certain species of free fatty acids (FFAs), have the potential to be identified by the lipid translocator CD36, while neuropeptide stimulators are believed to be recognized by their corresponding neuropeptide receptors (NPRs). Both of these mediators have the ability to enhance the expression of genes involved in immune responses, although the specific signaling pathways through which these receptors and immune response genes operate remain unclear. Prior to being released in an active state into the extracellular region, the inactive form of proinflammatory cytokines, such as pro-IL-1β, necessitates proteolytic processing. This processing is facilitated through the activation of the NLRP3 inflammasome complex. Subsequently, proteins of cytokines, AMPs, chemokines, and MMPs are secreted into the extracellular regions in order to regulate the functioning of neighboring cells, thereby resulting in a cascade of subsequent effects.