Abstract
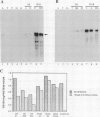
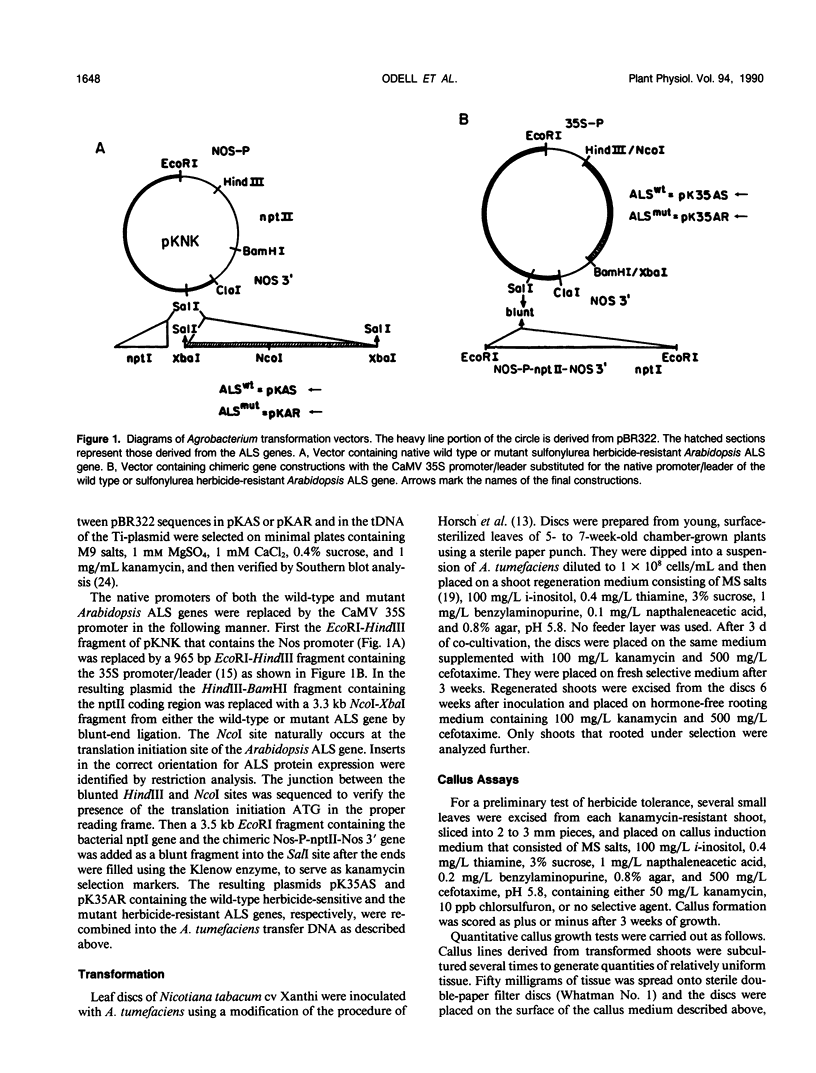
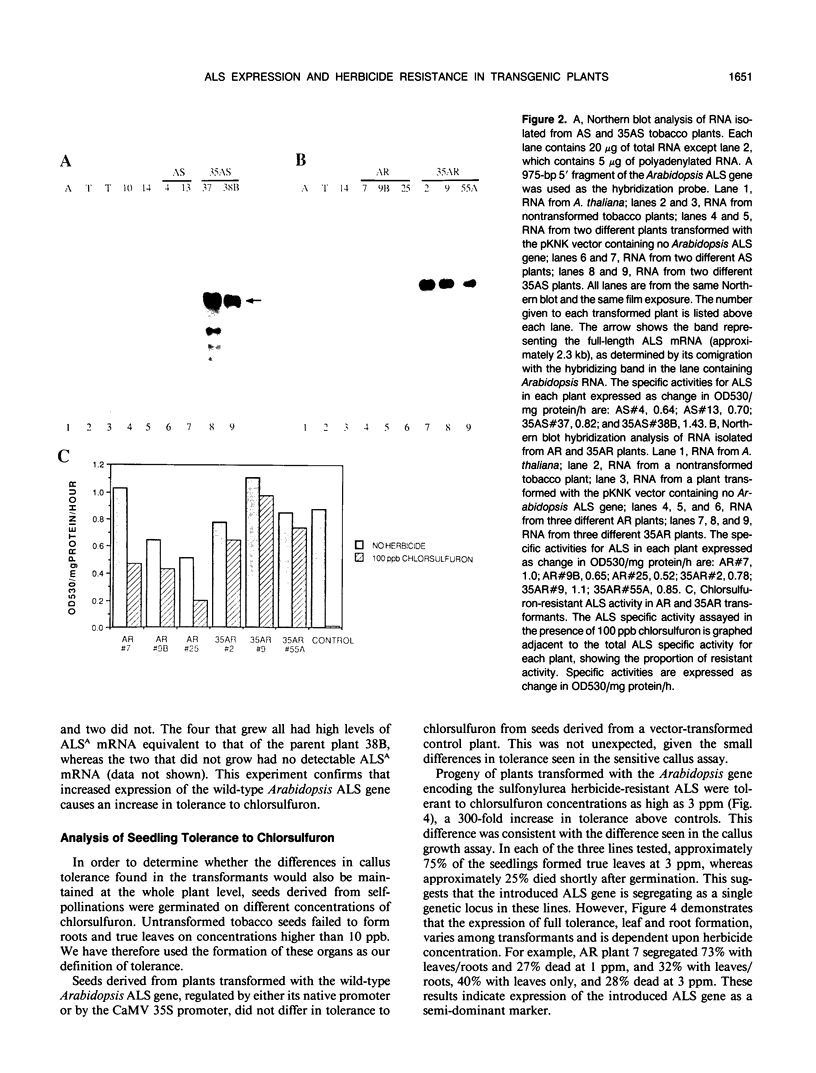
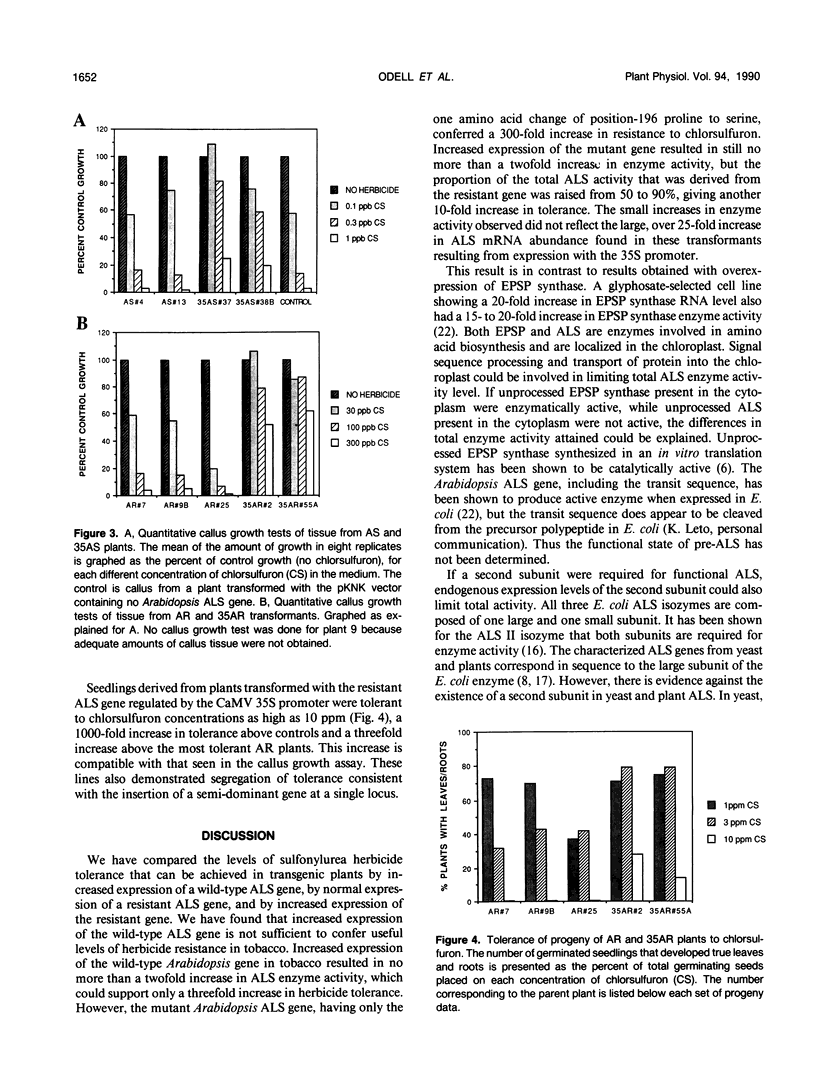
Genes encoding wild type acetolactate synthase (ALS) and a sulfonylurea herbicide-resistant form of the enzyme, isolated from Arabidopsis thaliana, were expressed in transgenic Nicotiana tabacum plants under the control of their native promoters or of the highly active cauliflower mosaic virus 35S promoter. Expression of the wild type coding region from the 35S promoter resulted in a small, threefold increase in sulfonylurea tolerance above the levels measured in tissue expressing the native wild type gene. A much larger, 300-fold increase in herbicide tolerance was conferred by the mutant gene encoding a herbicide-resistant ALS. An additional 10-fold increase in tolerance was attained by expressing this coding region from the 35S promoter. The increase in both wild type and mutant gene expression directed by the 35S promoter resulted in over 25-fold higher levels of ALS messenger RNA in some transformants as compared with those expressing the native genes. However, ALS specific activity increased at most twofold, indicating that the amount of functional enzyme and messenger RNA are not correlated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaleff R. S., Mauvais C. J. Acetolactate synthase is the site of action of two sulfonylurea herbicides in higher plants. Science. 1984 Jun 29;224(4656):1443–1445. doi: 10.1126/science.224.4656.1443. [DOI] [PubMed] [Google Scholar]
- Chaleff R. S., Ray T. B. Herbicide-resistant mutants from tobacco cell cultures. Science. 1984 Mar 16;223(4641):1148–1151. doi: 10.1126/science.223.4641.1148. [DOI] [PubMed] [Google Scholar]
- Della-Cioppa G., Bauer S. C., Klein B. K., Shah D. M., Fraley R. T., Kishore G. M. Translocation of the precursor of 5-enolpyruvylshikimate-3-phosphate synthase into chloroplasts of higher plants in vitro. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6873–6877. doi: 10.1073/pnas.83.18.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S. Genetic analysis of mutants of Saccharomyces cerevisiae resistant to the herbicide sulfometuron methyl. Genetics. 1985 Jan;109(1):21–35. doi: 10.1093/genetics/109.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S., Livak K. J. Nucleotide sequence of the yeast ILV2 gene which encodes acetolactate synthase. Nucleic Acids Res. 1985 Jun 11;13(11):4011–4027. doi: 10.1093/nar/13.11.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., Ma Y., Buchbinder B. U., Pyne J. W., Sun S. M., Bliss F. A. Messenger RNA for G1 protein of French bean seeds: Cell-free translation and product characterization. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3196–3200. doi: 10.1073/pnas.75.7.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. Y., Townsend J., Tepperman J., Black M., Chui C. F., Mazur B., Dunsmuir P., Bedbrook J. The molecular basis of sulfonylurea herbicide resistance in tobacco. EMBO J. 1988 May;7(5):1241–1248. doi: 10.1002/j.1460-2075.1988.tb02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Odell J. T., Schreiner R. M. Soybean protoplast culture and direct gene uptake and expression by cultured soybean protoplasts. Plant Physiol. 1987 Jul;84(3):856–861. doi: 10.1104/pp.84.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M. F., Umbarger H. E. Effects of deletion and insertion mutations in the ilvM gene of Escherichia coli. J Bacteriol. 1987 Feb;169(2):600–604. doi: 10.1128/jb.169.2.600-604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F., Smith J. K. Isolation and characterization of plant genes coding for acetolactate synthase, the target enzyme for two classes of herbicides. Plant Physiol. 1987 Dec;85(4):1110–1117. doi: 10.1104/pp.85.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Horsch R. B., Klee H. J., Kishore G. M., Winter J. A., Tumer N. E., Hironaka C. M., Sanders P. R., Gasser C. S., Aykent S., Siegel N. R., Rogers S. G., Fraley R. T. Engineering herbicide tolerance in transgenic plants. Science. 1986 Jul 25;233(4762):478–481. doi: 10.1126/science.233.4762.478. [DOI] [PubMed] [Google Scholar]
- Smith J. K., Schloss J. V., Mazur B. J. Functional expression of plant acetolactate synthase genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4179–4183. doi: 10.1073/pnas.86.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stalker D. M., Hiatt W. R., Comai L. A single amino acid substitution in the enzyme 5-enolpyruvylshikimate-3-phosphate synthase confers resistance to the herbicide glyphosate. J Biol Chem. 1985 Apr 25;260(8):4724–4728. [PubMed] [Google Scholar]
- Williamson J. D., Galili G., Larkins B. A., Gelvin S. B. The synthesis of a 19 kilodalton zein protein in transgenic petunia plants. Plant Physiol. 1988 Dec;88(4):1002–1007. doi: 10.1104/pp.88.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav N., McDevitt R. E., Benard S., Falco S. C. Single amino acid substitutions in the enzyme acetolactate synthase confer resistance to the herbicide sulfometuron methyl. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4418–4422. doi: 10.1073/pnas.83.12.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P., Joos H., Genetello C., Leemans J., Montagu M. V., Schell J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983;2(12):2143–2150. doi: 10.1002/j.1460-2075.1983.tb01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]