Abstract
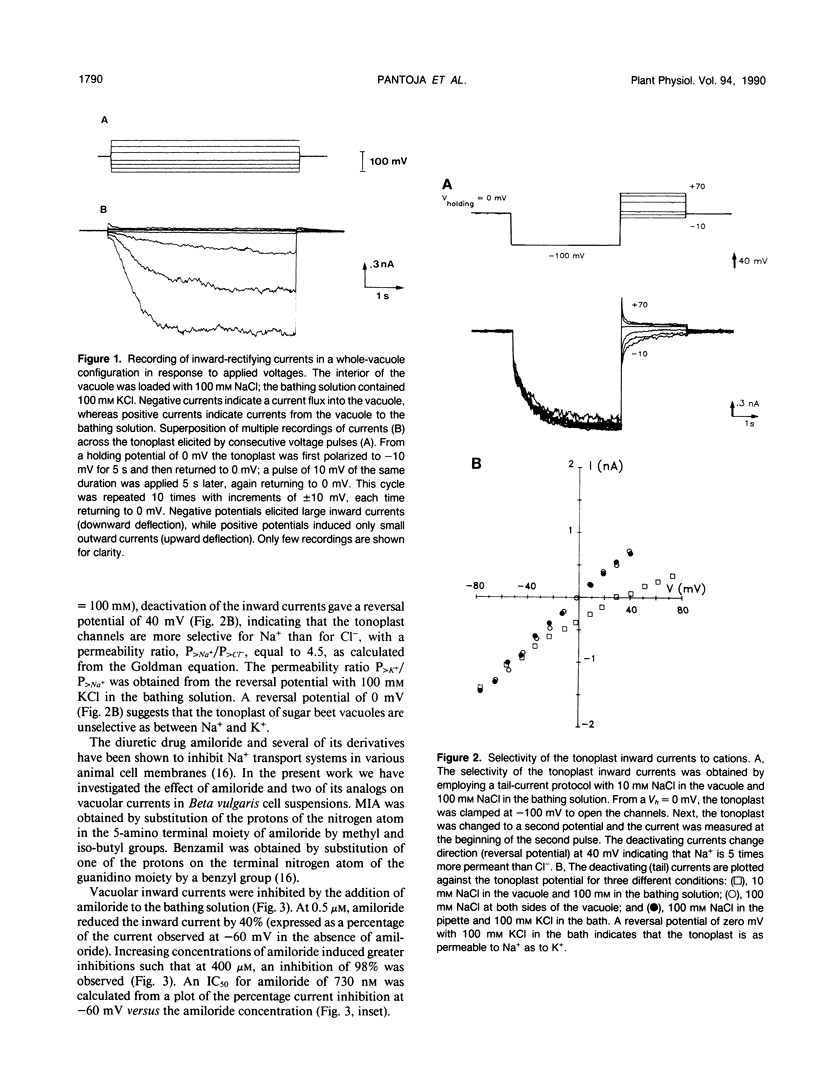
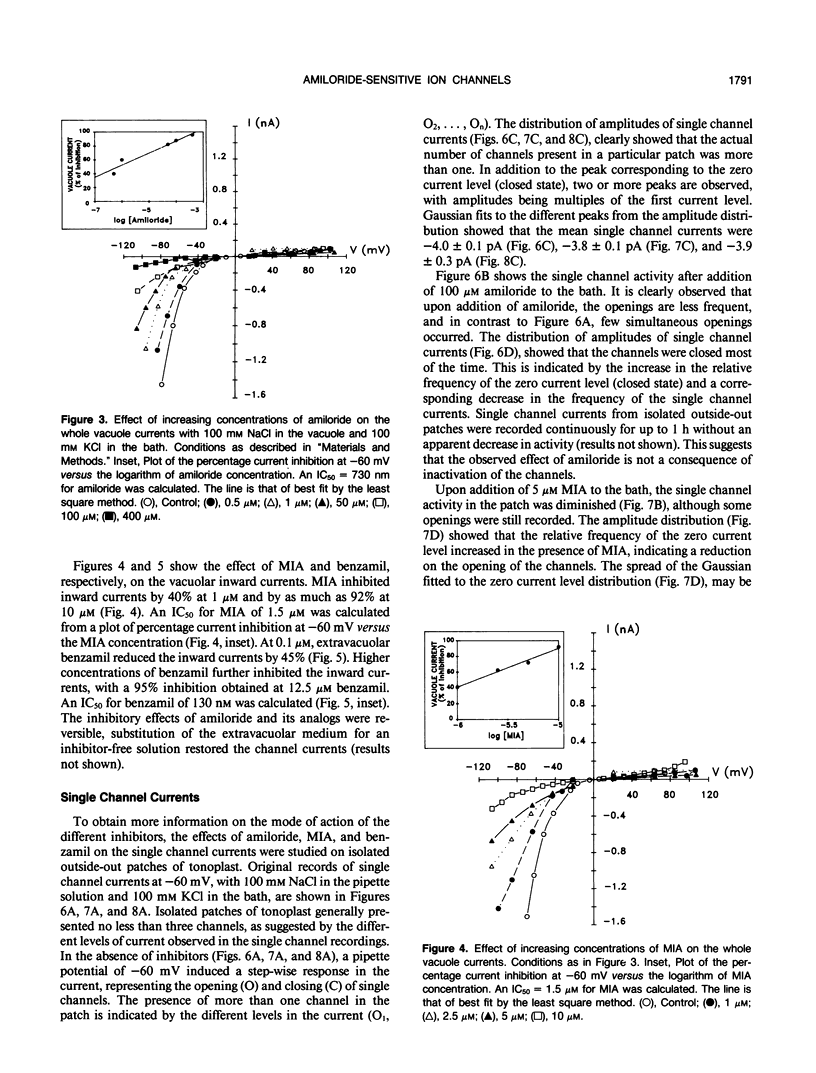
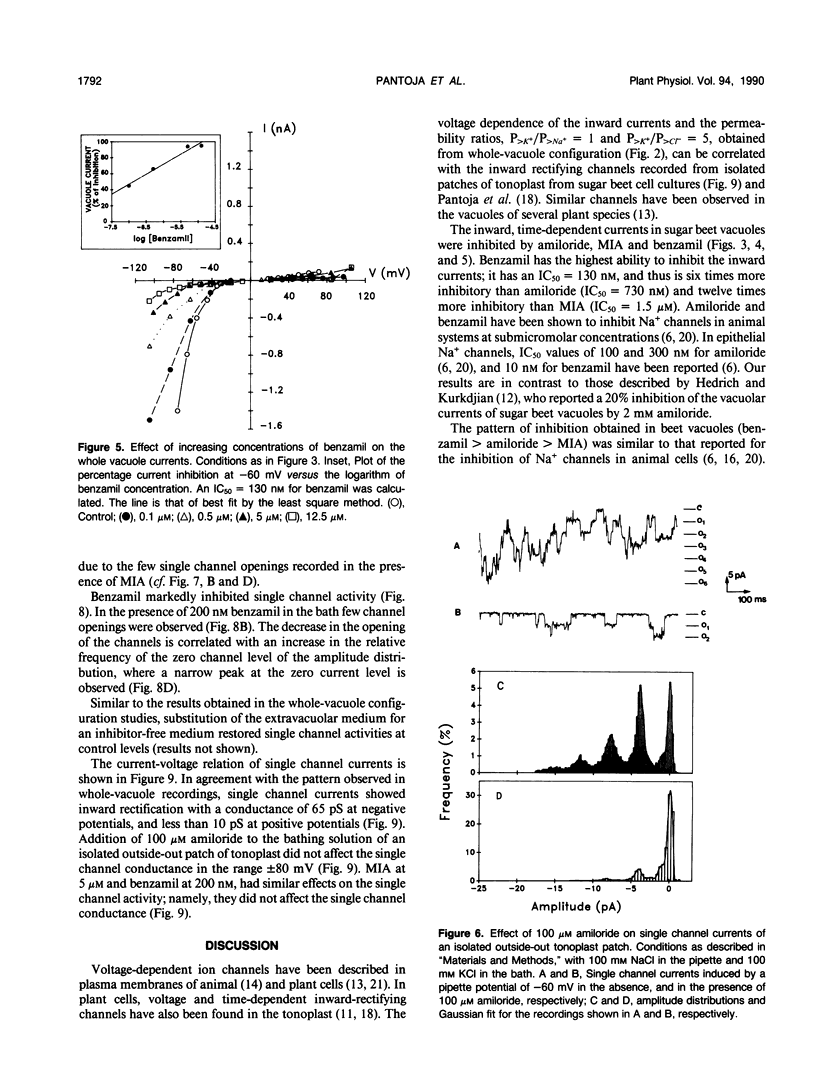
The properties of the vacuolar membrane (tonoplast) ion channels of sugar beet (Beta vulgaries) cell cultures were studied using the patch-clamp technique. Tonoplast currents displayed inward rectification in the whole vacuole and isolated outside-out patch configurations and permeability ratios PK+/PNa+ = 1 and PK+/PCl− = 5. Amiloride and two of its analogs, 5-(N-methyl-N-isobutyl)-amiloride and benzamil, inhibitors of Na+ channels in animal systems, blocked inward currents by reducing single-channel openings. Concentrations for 50% inhibition of vacuolar currents of 730 nanomolar, 130 nanomolar, and 1.5 micromolar for amiloride, benzamil, and 5-(N-methyl-N-isobutyl)-amiloride, respectively, were obtained from whole-vacuole recordings. The high inhibitory action (affinity) of amiloride and its analogs for the tonoplast cation channel suggests that these compounds could be used for the isolation and biochemical characterization of this protein.
Full text
PDF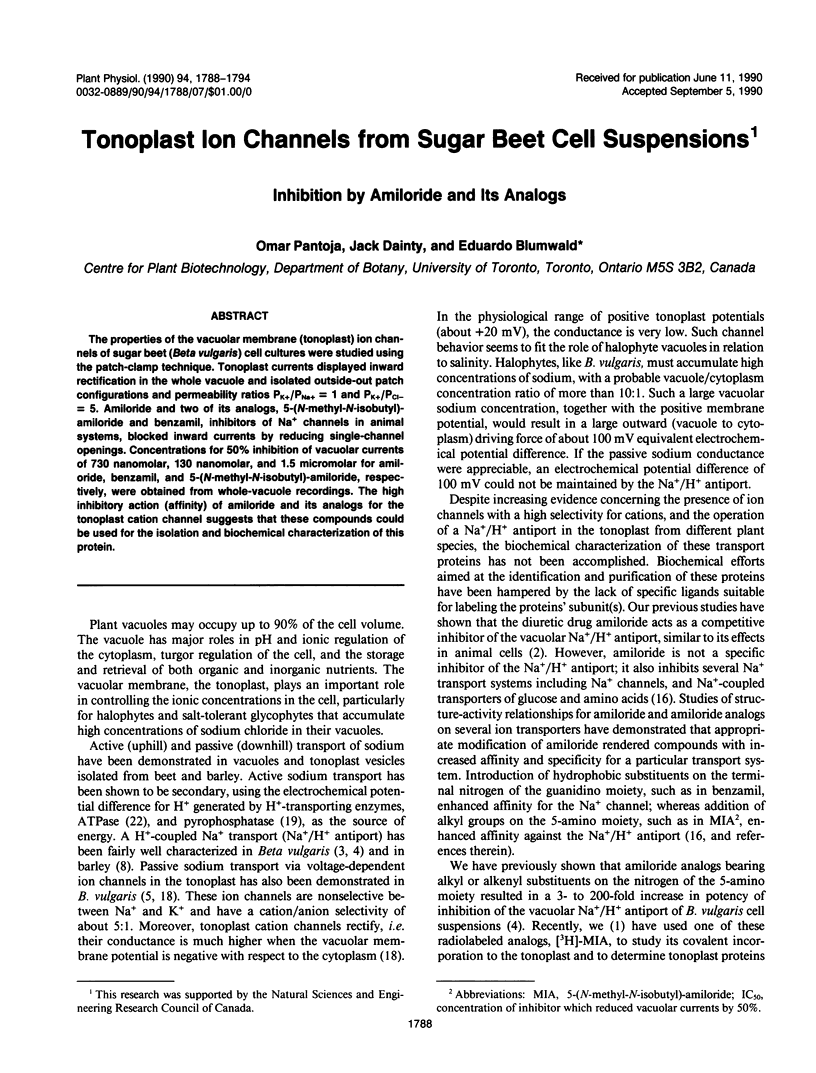


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkla B. J., Charuk J. H., Cragoe E. J., Blumwald E. Photolabeling of tonoplast from sugar beet cell suspensions by [h]5-(N-methyl-N-isobutyl)-amiloride, an inhibitor of the vacuolar na/h antiport. Plant Physiol. 1990 Jul;93(3):924–930. doi: 10.1104/pp.93.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Cragoe E. J., Poole R. J. Inhibition of na/h antiport activity in sugar beet tonoplast by analogs of amiloride. Plant Physiol. 1987 Sep;85(1):30–33. doi: 10.1104/pp.85.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Na/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985 May;78(1):163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Salt tolerance in suspension cultures of sugar beet : induction of na/h antiport activity at the tonoplast by growth in salt. Plant Physiol. 1987 Apr;83(4):884–887. doi: 10.1104/pp.83.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert A. W., Fanelli G. M. Effects of some pyrazinecarboxamides on sodium transport in frog skin. Br J Pharmacol. 1978 May;63(1):139–149. doi: 10.1111/j.1476-5381.1978.tb07783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Barbry P., Vigne P., Chassande O., Cragoe E. J., Jr, Lazdunski M. Amiloride and its analogs as tools to inhibit Na+ transport via the Na+ channel, the Na+/H+ antiport and the Na+/Ca2+ exchanger. Biochimie. 1988 Sep;70(9):1285–1290. doi: 10.1016/0300-9084(88)90196-4. [DOI] [PubMed] [Google Scholar]
- Garbarino J., Dupont F. M. Rapid induction of na/h exchange activity in barley root tonoplast. Plant Physiol. 1989 Jan;89(1):1–4. doi: 10.1104/pp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton K. L., Eaton D. C. Single-channel recordings from amiloride-sensitive epithelial sodium channel. Am J Physiol. 1985 Sep;249(3 Pt 1):C200–C207. doi: 10.1152/ajpcell.1985.249.3.C200. [DOI] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2767–2770. doi: 10.1073/pnas.83.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban-Sohraby S., Benos D. J. The amiloride-sensitive sodium channel. Am J Physiol. 1986 Feb;250(2 Pt 1):C175–C190. doi: 10.1152/ajpcell.1986.250.2.C175. [DOI] [PubMed] [Google Scholar]
- Schroeder J. I., Hedrich R. Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem Sci. 1989 May;14(5):187–192. doi: 10.1016/0968-0004(89)90272-7. [DOI] [PubMed] [Google Scholar]


