Abstract
We present a female patient with heart failure with reduced ejection fraction who underwent left bundle branch cardiac resynchronization therapy. Left bundle branch lead implantation was complicated with septal branch perforation causing an iatrogenic coronary fistula complicated by septal hematoma formation and development of shock. Occlusion by covered stents was successfully achieved.
Key Words: acute heart failure, cardiac resynchronization therapy, coronary angiography, percutaneous coronary intervention
Graphical abstract
History of Presentation
A 61-year-old female patient with recent diagnosis of dilated cardiomyopathy, heart failure with reduced ejection fraction (23%) in stage C, and left bundle branch block (180 ms) on electrocardiography was admitted to the hospital. Her coronary angiography (CA) demonstrated normal coronary arteries without any coronary malformation.
Learning objectives
-
•
To describe an unusual angioplasty to solve a rare complication of IVSH following LOT-CRT.
-
•
To provide recommendations regarding how to recognize and plan its treatment.
-
•
To comment on the published therapeutic options.
Due to hypotension by dosing up of neurohormonal block, the heart team multidisciplinary team meeting decided on left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT). During the procedure and after achieving an adequate left bundle branch lead placement, a sudden increase in the pace threshold level was observed, so a second attempt was carried out failing to achieve left bundle branch pacing (LBBP). A third attempt successfully achieved adequate parameters. She was discharged on the next day, with an electrocardiogram that showed a 128-ms QRS stimulation, and without adverse events.
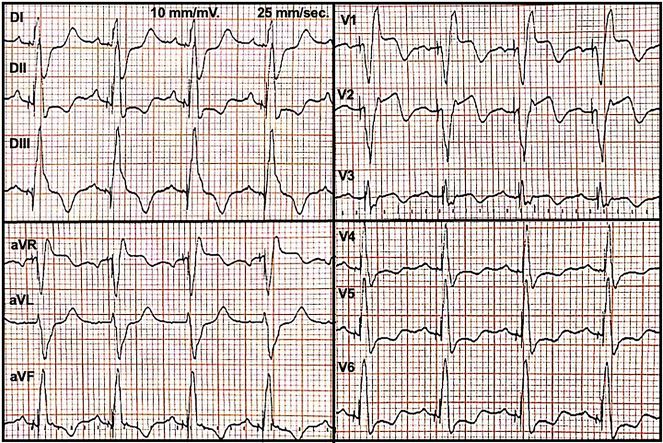
After 2 days of discharge, she was admitted because of sudden chest pain and shortness of breath. Vital signs demonstrated blood pressure 105/70 mm Hg, heart rate 60 beats/min, oxygen saturation 96% on room air, and normal temperature. On physical examination, jugular venous pressure was normal and heart auscultation was normal. Electrocardiography revealed paced QRS complex with slight ST-segment elevation in aVR-V1-V2 leads (Figure 1), and a laboratory test highlighted an increase of troponin up to 6,319 ng/mL (normal value up to 13 ng/mL).
Figure 1.
Admission Electrocardiogram
Question 1: What Is the Differential Diagnosis, and Which Initial Test Would Be the Test of Choice?
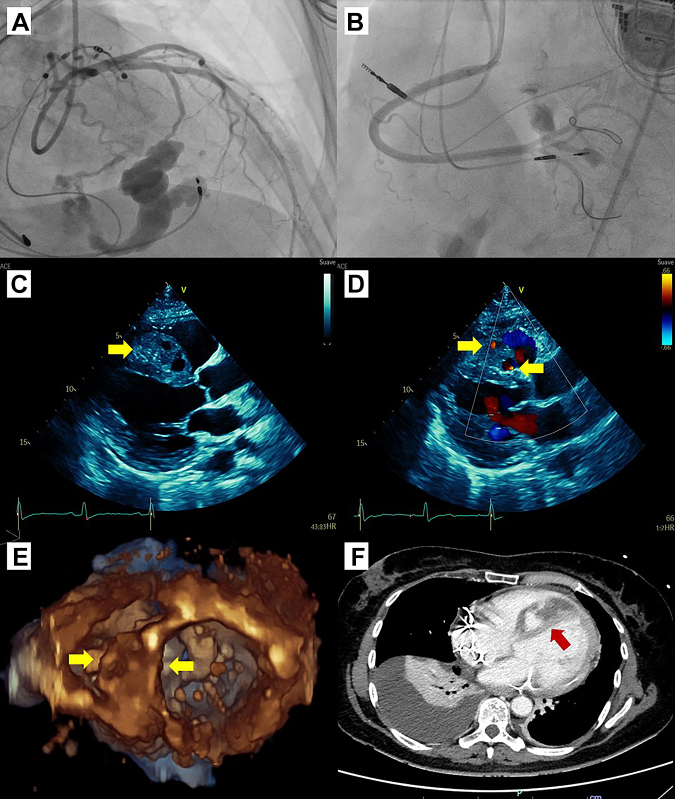
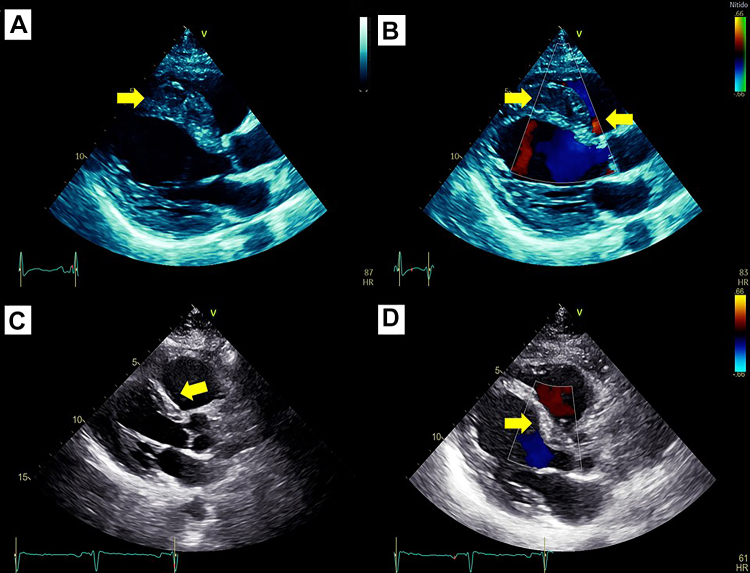
Answer: Given the clinical history after LOT-CRT, the differential diagnoses included pneumothorax, cardiac tamponade, acute heart failure, or pulmonary embolism. We performed a chest radiography, which showed mild pulmonary congestion, and pneumothorax was excluded. Thereafter, we wanted to rule out cardiac tamponade by echocardiography. However, echocardiography highlighted a 25 × 60 mm interventricular septal hematoma (IVSH) associated with active flow by color Doppler (Figure 2).
Figure 2.
Initial Diagnostic Tests
(A) Fistula from the first and second septal branches of the left anterior descending artery. (B) Fistula from the septal branch of the posterior descending artery. (C) Interventricular septal hematoma (IVSH) by parasternal long-axis view (arrow). (D) Flow by color Doppler inside of IVSH (arrow). (E) Three-dimensional echocardiogram of IVSH (arrow). (F) Computed tomography angiography shows the IVSH (arrow).
Subsequently, ST-segment elevation myocardial infarction had to be excluded, and an urgent CA was performed, which excluded obstructive coronary artery disease, but 3 coronary fistulas were identified: one fistula from the first septal branch of the left anterior descending artery (LAD) to the coronary sinus; a second one from the second septal branch of the LAD to the interventricular septum; and a third one that went from a secondary branch of the posterior descending artery (PD) to the interventricular septum (Figure 2). The patient then was transferred to the intensive care unit, stable and without chest pain. The patient then had a computed tomography scan, which confirmed the presence of an IVSH (142 mm × 70 mm × 34 mm) from septal branches, mild hemopericardium effusion, and moderate right-sided pleural effusion (Figure 2).
Question 2: What Are the General Causes of IVSH, and What Is the Reason of This Hematoma?
Answer: IVSH is a rare complication that has been described during interventional procedures, such as percutaneous coronary artery intervention, cardiac revascularization, after closing a ventricular septal defect, and recently a few reports after LBBP.1, 2, 3, 4
It has been hypothesized that directed injury to the septal branches of the coronary arteries due to an invasive procedure in proximity to the interventricular septum. In our case, septal branches were perforated iatrogenically after 3 attempts of achieving LBBP. Subsequently, after septal branch perforation, a hematoma was progressively developed.1, 2, 3, 4
Question 3: What Are the Clinical Consequences of IVSH?
Answer: Within 48 hours, the patient progressively developed sinus tachycardia and third tone on cardiac auscultation, blood pressure dropped nearly 90/60 mm Hg, peripheral vasoconstriction, and blood test showed lactic acidosis (pH 7.33, bicarbonate 19 mmol/L and lactate 2.6 mmol/L), and hemoglobin was stable in 10 g/dL, and aminotransferase in 101 U/L. In bedside echocardiography, pericardial effusion was stable, cardiac output was 3.3 L/min, the inferior vena cava sized up 2.2 cm, B lines were present, and there was no evidence of outflow tract obstruction. She was classified in cardiogenic shock (Society for Cardiovascular Angiography and Interventions Cardiogenic Shock Working Group stage B) and was started on low dose of milrinone.
The natural history of IVSH is subendocardial bleeding with the development and further expansion of the hematoma and progressive worsening of right and left ventricular systolic and diastolic function. Subsequently, filling pressures rise and cardiac output decreases. Even myocardial rupture, thrombus formation, abscess formation, right and left ventricular outflow tract obstruction, conduction system abnormalities, cardiac tamponade, and risk for multiorgan dysfunction and mortality have been described in the literature.1,2
Question 4: What Are the Therapeutic Options in Cases Like This?
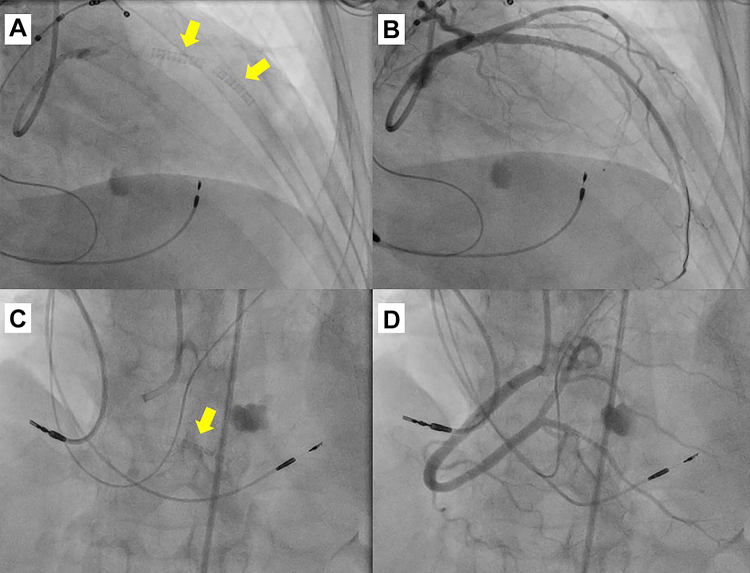
Answer: Due to progressive hemodynamic decompensation, the heart team decided a percutaneous resolution by covered stent. Consequently, an angioplasty of 2 expanded polytetrafluoroethylene-covered GraftMaster 3.5 × 16 mm and 2.8 × 16 mm stents (Abbott Laboratories) in the LAD was performed, closing 2 fistulas. Thereafter, another 2.8 × 16 mm covered stent was placed in the PD (Figure 3, Video 1).
Figure 3.
Coronary Angioplasty and Results
Coronary angiography shows the placement of polytetrafluoroethylene-covered stents in the left anterior descending artery and posterior descending artery to close respective fistulas. (A) Stents (arrow) in the left anterior descending artery. (B) Absence of flow through fistulas. (C) Stent (arrow) in branch of posterior descending artery. (D) Absence of flow through fistula.
With regard to the literature, there has been a lack of consensus on the management of IVSH secondary to LOT-CRP. A benign evolution has been published, especially in asymptomatic cases.3 The therapeutic approach for symptomatic patients is based on size, hemodynamic context, and etiology. Published data on successful treatment of IVSH after LBBP have described conservative conductance,3,4 metallic spring coil implantation,5 repositioning lead,6 and coronary embolization.7 In this way, the treatment spectrum is wide, ranging from expected management to interventional treatment.
In our case, with the detection of multiple coronary fistulas and development of cardiogenic shock, covered stents placement was a novel treatment option to halt the fistula’s flow, and their metallic meshwork also covered the coronary perforation. Indeed, there are data showing how useful covered stent implantation is in fistula treatment.8,9 We would rather place polytetrafluoroethylene-covered stents instead of coil placement because there were 2 big fistulas from the LAD, and if there had been any coil procedure complication, the patient would have been in danger of losing LAD flow.
Question 5: Are There Any Options to Prevent, and How to Follow-Up This Unusual Complication?
Answer: Early detection of this complication is crucial. Analyzing CA before LOT-CRP procedure could be helpful to select the safest lead position, and even using fusion images in complex cases. During LOT-CRP, if it is a difficult procedure or associated with several attempts, a CA should be considered to discard a septal branch perforation. Additionally, a sudden increase of pace threshold level of the left bundle branch lead should lead to suspicion of this complication. After LOT-CRP, a routine echocardiogram is useful to evaluate any complication related to interventricular septum.
In parallel, output of pacemaker electrode must be checked days after. In fact, we noticed the output of the LBBP electrode increased owing to IVSH and decreased after its partial reabsorption.
Long dual antiplatelet therapy (DAPT) was prescribed, which is the preferred choice because of the disadvantages regarding thrombogenicity and stent-related complications of using covered stents. Adding anticoagulation could be an option; however, to the best of our knowledge, there is no randomized trial to evaluate DAPT alone vs anticoagulant alone and/or DAPT plus anticoagulant.10
After treatment, it is also important to check any improvement, particularly absence of flow inside the IVSH, functional class, and heart failure status. In our patient, echocardiography 7 days after angioplasty showed a partial reduction in the IVSH size, no flow by color Doppler, and no progression of the pericardial effusion (Figure 4, Video 2). After 6 months, a new echocardiogram highlighted ejection fraction improvement up to 40% and the IVSH disappearance (Figure 4). As a follow-up, she is now in NYHA functional class I with substantial clinical improvement.
Figure 4.
Follow-up Echocardiogram
Echocardiography 7 days after angioplasty shows (A) that the interventricular septal hematoma decreased size (arrow) and (B) no flow by color Doppler (arrow). After 6 months: (C) highlighted absence of interventricular septal hematoma (arrow) and (D) no flow inside of septum (arrow).
Conclusions
IVSH is a rare but potentially adverse event after LOT-CRP. Patient monitoring after invasive procedures is mandatory, and our group decided to perform an echocardiogram after every LBBP or LOT-CRP placement to watch this complication. The acute clinical onset must be a sign of alarm, and progressive worsening and larger-size hematoma are key points to decide an interventional approach. Cardiac imaging is crucial in the diagnosis and management. To the best of our knowledge, this is the first case report of multiple fistula secondary to LOT-CRT procedure treated with covered stents, with excellent clinical outcomes.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Coronarography and angioplasty.
Echocardiogram before and after 7 days of coronary angioplasty.
References
- 1.Wang Y., Ma D., Zhang B., et al. Myocardial contrast echocardiographic diagnosis and follow-up of interventricular septal hematoma after retrograde intervention for a chronic total occlusion of a right coronary artery: a case report. Cardiovasc Diagn Ther. 2022;12(2):253–261. doi: 10.21037/cdt-21-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu B., Zhou Y., Lu J., et al. Interventricular septal hematoma caused by percutaneous intramyocardial septal radiofrequency ablation successfully treated with coil embolization. J Am Coll Cardiol Intv. 2023;16(6):722–724. doi: 10.1016/j.jcin.2022.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi R., Rattigan E., Bauch T.D., et al. Giant interventricular septal hematoma complicating left bundle branch pacing: a cautionary tale. J Am Coll Cardiol Case Rep. 2023;16 doi: 10.1016/j.jaccas.2023.101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng R., Wu S., Wang S., et al. Case report: interventricular septal hematoma complicating left bundle branch pacing lead implantation. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.744079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X., Lu H., Xu L., et al. Interventricular septal hematoma with pericardium effusion after left bundle branch pacing implantation. J Am Coll Cardiol EP. 2023;9(1):142–144. doi: 10.1016/j.jacep.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari ADL, Klafke LH, Soccol R, et al. Coronary artery complications after left bundle branch area pacing: an increasingly reported issue in the era of physiologic pacing. Pacing Clin Electrophysiol. Published online May 9, 2023. https://doi.org/10.1111/pace.14710 [DOI] [PubMed]
- 7.Huang W., Zheng R., Wu S. B-O02-212-interventricular septal hematoma complicating left bundle branch pacing lead implantation. Heart Rhythm. 2021;18(8):S185. doi: 10.3389/fcvm.2021.744079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassaian S.E., Mahmoodian M., Salarifar M., et al. Stent-graft exclusion of multiple symptomatic coronary artery fistulae. Tex Heart Inst J. 2007;34(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- 9.Mestre Barceló J.L., Salido Tahoces L., del Río del Busto A., et al. Closure of an iatrogenic coronary artery fistula with a PTFE-coated stent. Rev Esp Cardiol. 2004;57(7):699–701. [PubMed] [Google Scholar]
- 10.Mikhail P., Howden N., Monjur M., et al. Coronary perforation incidence, outcomes and temporal trends (COPIT): a systematic review and metaanalysis. Open Heart. 2022;9 doi: 10.1136/openhrt-2022-002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronarography and angioplasty.
Echocardiogram before and after 7 days of coronary angioplasty.