Abstract
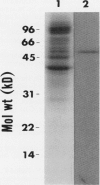
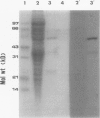
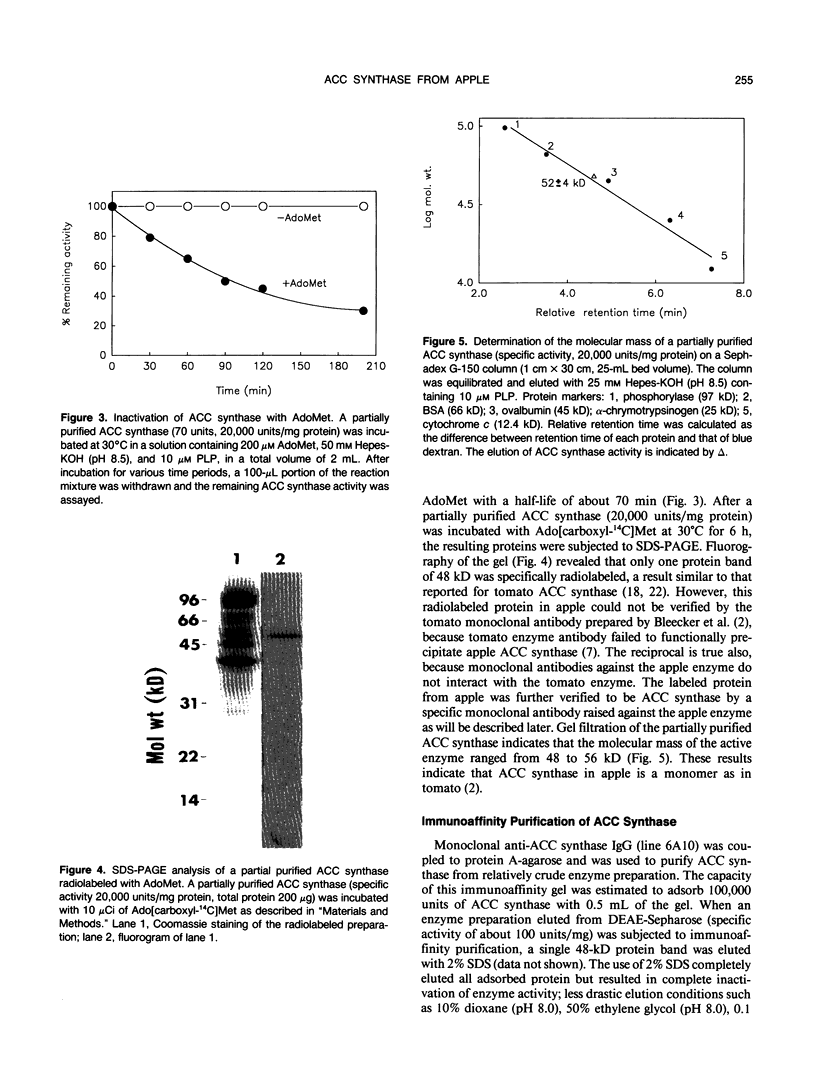
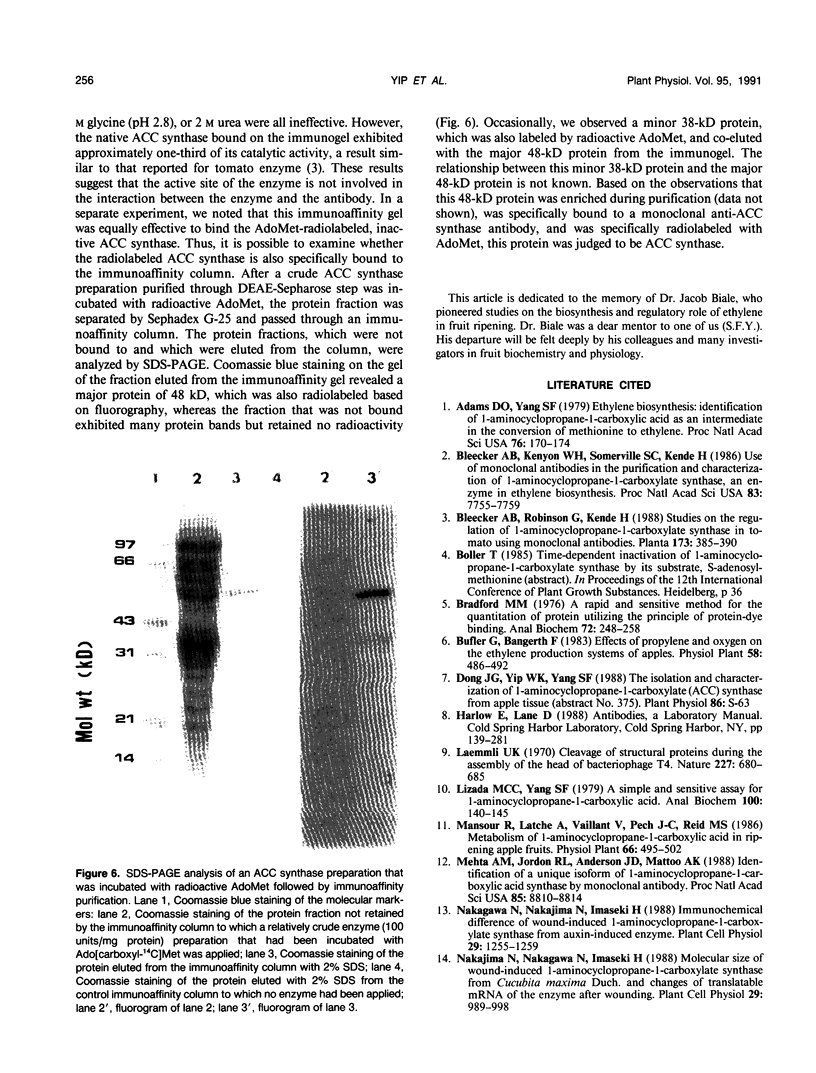
1-Aminocyclopropane-1-carboxylate (ACC) synthase, a key enzyme in ethylene biosynthesis, was isolated and partially purified from apple (Malus sylvestris Mill.) fruits. Unlike ACC synthase isolated from other sources, apple ACC synthase is associated with the pellet fraction and can be solubilized in active form with Triton X-100. Following five purification steps, the solubilized enzyme was purified over 5000-fold to a specific activity of 100 micromoles per milligram protein per hour, and its purity was estimated to be 20 to 30%. Using this preparation, specific monoclonal antibodies were raised. Monoclonal antibodies against ACC synthase immunoglobulin were coupled to protein-A agarose to make an immunoaffinity column, which effectively purified the enzyme from a relatively crude enzyme preparation (100 units per milligram protein). As with the tomato enzyme, apple ACC synthase was inactivated and radiolabeled by its substrate S-adenosyl-l-methionine. Apple ACC synthase was identified to be a 48-kilodalton protein based on the observation that it was specifically bound to immunoaffinity column and it was specifically radiolabeled by its substrate S-adenosyl-l-methionine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker A. B., Kenyon W. H., Somerville S. C., Kende H. Use of monoclonal antibodies in the purification and characterization of 1-aminocyclopropane-1-carboxylate synthase, an enzyme in ethylene biosynthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7755–7759. doi: 10.1073/pnas.83.20.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Mehta A. M., Jordan R. L., Anderson J. D., Mattoo A. K. Identification of a unique isoform of 1-aminocyclopropane-1-carboxylic acid synthase by monoclonal antibody. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8810–8814. doi: 10.1073/pnas.85.23.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves H. C., Heeren R., Malloy P. Enzyme purification using antibody crosslinked to protein A agarose: application to Escherichia coli NADP-isocitrate dehydrogenase. Anal Biochem. 1981 Jul 15;115(1):194–196. doi: 10.1016/0003-2697(81)90545-5. [DOI] [PubMed] [Google Scholar]
- Sato T., Theologis A. Cloning the mRNA encoding 1-aminocyclopropane-1-carboxylate synthase, the key enzyme for ethylene biosynthesis in plants. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6621–6625. doi: 10.1073/pnas.86.17.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S., Yang S. F. S-adenosylmethionine-dependent inactivation and radiolabeling of 1-aminocyclopropane-1-carboxylate synthase isolated from tomato fruits. Plant Physiol. 1988 Sep;88(1):109–114. doi: 10.1104/pp.88.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S., Yang S. F. Specificity of S-adenosyl-L-methionine in the inactivation and the labeling of 1-aminocyclopropane-1-carboxylate synthase isolated from tomato fruits. Arch Biochem Biophys. 1989 May 15;271(1):107–112. doi: 10.1016/0003-9861(89)90260-9. [DOI] [PubMed] [Google Scholar]
- Tsai D. S., Arteca R. N., Bachman J. M., Phillips A. T. Purification and characterization of 1-aminocyclopropane-1-carboxylate synthase from etiolated mung bean hypocotyls. Arch Biochem Biophys. 1988 Aug 1;264(2):632–640. doi: 10.1016/0003-9861(88)90329-3. [DOI] [PubMed] [Google Scholar]
- Van der Straeten D., Van Wiemeersch L., Goodman H. M., Van Montagu M. Cloning and sequence of two different cDNAs encoding 1-aminocyclopropane-1-carboxylate synthase in tomato. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4859–4863. doi: 10.1073/pnas.87.12.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Straeten D., Van Wiemeersch L., Goodman H. M., Van Montagu M. Purification and partial characterization of 1-aminocyclopropane-1-carboxylate synthase from tomato pericarp. Eur J Biochem. 1989 Jul 1;182(3):639–647. doi: 10.1111/j.1432-1033.1989.tb14873.x. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]