Figure 2.
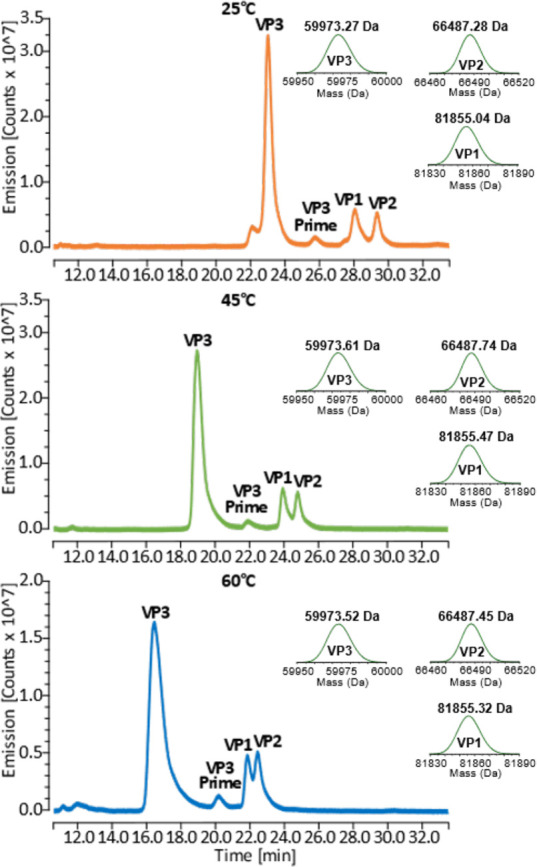
A comparison of HEK293-derived full-length AAV2 viral capsid protein (VP) separation profiles when separation is performed at column temperatures of 25 °C (top), 45 °C (middle), and 60 °C (bottom) during HILIC-FLR-MS using DFA as an ion pairing agent. Deconvoluted MS spectra of the unmodified VPs are shown on the right of each FLR trace. All samples were run in technical triplicate. Separation was performed on an Acquity UPLC glycoprotein BEH amide column, 300 Å, 1.7 μm, 2.1 × 150 mm with a gradient of 64.5–58.5% B. FLR traces were monitored by using λem = 280 and λex = 348 nm. Clear separation of all three viral proteins was seen at all column temperatures with better separation between VP2 and VP1 observed as column temperature decreased. An additional peak labeled VP3 Prime was also detected.
