Abstract
BACKGROUND:
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by multisystem inflammation. Medical management of SLE is based on reducing inflammation and tissue damage in the affected organs; however, medications used to treat SLE have been found to contribute to additional organ damage. Therefore, finding new ways to predict and prevent flares that require an inpatient (IP) stay or emergency department (ED) visit is critical for reducing the clinical and economic burden in patients with SLE.
OBJECTIVE:
To identify risk factors of SLE flares requiring an IP/ED visit among a Medicaid-insured population with SLE.
METHODS:
This retrospective study included patients from the Merative MarketScan Medicaid database (2013-2019). To capture patients at all stages of their SLE journey, all SLE claims for a patient were captured, and the index date was randomly selected among those claims that were at least 12 months after the first evidence of SLE. Patients were required to be continuously enrolled 1-year pre-index (year 1) and post-index (year 2). Demographics, clinical characteristics, and health care use and costs were measured in year 1, and flares requiring an IP/ED visit were identified in year 2 using the Garris algorithm. Multivariable logistic regression and classification and regression tree (CART) modeling were used to identify year 1 predictors and combination of factors, respectively, associated with flares-related IP/ED visits.
RESULTS:
Of the 8,083 patients included in the study, 37.6% of patients (n = 3,039) had a flare. Logistic regression identified ED visits in year 1 as one of the strongest predictors of flares-related IP/ED visits in year 2 (odds ratio = 2.19 [95% CI = 1.93-2.49]). SLE treatment progression to biologics (0.54 [0.42-0.70]) was the strongest predictor of decreased odds. Other strong predictors included other neurological disorders (1.63 [1.43-1.87]), Black race (1.49 [1.32-1.68]), chronic kidney disease/renal failure (1.35 [1.10-1.66]), and opioid use (1.30 [1.17-1.45]). CART modeling identified patients with an ED visit, an IP admission, and a diagnosis of Elixhauser Comorbidity Index–defined other neurological disorders in year 1 as having the highest probability of a flare-related IP/ED visit in year 2 (probability = 0.708), whereas patients without an ED visit had the lowest probability (probability = 0.185).
CONCLUSIONS:
Patients with the highest risk of a flare that required an IP/ED visit were those with a prior ED visit, IP admission, and other neurological disorders. Modeling also identified patients with prior opioid use, Black patients, and patients without SLE medications as subgroups with a high risk of a flare requiring an IP/ED visit.
Plain language summary
The results from this study identified groups of patients with systemic lupus erythematosus at higher risk of a flare-related inpatient (IP) stay/emergency department (ED) visit. Patients who had a prior ED visit or IP hospital stay in the prior year had the highest risk of a flare. Opioids and neurological disorders also increase the risk. There was a decreased risk among patients who were on a biologic medication. These results can be used to improve patient outcomes and reduce health care use and costs.
Implications for managed care pharmacy
This study identifies individual predictors (eg, opioid use and Black race) as well as combinations of risk factors (prior ED visits, IP stays, or other neurological disorders) that significantly increase the likelihood of having a flare-related IP/ED visit in the next year. This study also identifies the subgroups of patient with a particularly high probability for flare-related IP/ED visits and may provide the basis for more targeted disease management activities and input for clinical decision-making.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with an estimated prevalence of 241 per 100,000 people in the United States.1-3 SLE is characterized by multisystem inflammation affecting the skin, joints, kidneys, lungs, central nervous system, and hematopoietic system. It also has a relapsing–remitting course with cycles of lower disease status and periodic episodes of more active disease (ie, flares). Flares can increase in frequency and/or severity with disease progression.4
Medical management of SLE is based on reducing inflammation with the goal of preventing organ-damaging flares. The SLE treatment guidelines of the European Alliance of Associations for Rheumatology in 2019 recommend antimalarials for all patients with lupus. Glucocorticoids can provide rapid symptom relief, but the medium- to long-term aim should be minimized. Appropriate initiation of immunosuppressive drugs can expedite the tapering/discontinuation of glucocorticoids. Biologic agents should be considered for disease with inadequate control.3 However, long-term use of some of these medications may contribute to organ damage that can increase the risk of a flare and reduce therapy adherence.5
Previous research has explored clinical and biomarker predictors of flares, with little consistency.5,6 Although there have been some clinical factors and biomarkers that have demonstrated their use in clinical practice, they do not consistently identify the disease activity indicative of increasing flare risk in a generalizable population, and improving their predictive capabilities remains an area of ongoing research.5,6 Furthermore, many promising clinical factors and biomarkers are not routinely collected and may impose an additional burden in the routine clinical workflow.
SLE flares that result in an inpatient (IP) admission or emergency department (ED) visit place a large clinical and economic burden on both patients and health care systems vs more mild flares that can be treated in the outpatient (OP) setting. Therefore, it is critical to focus flare prevention efforts on these more severe flares. The objective of this study was to identify predictors of those SLE flares requiring an IP/ED visit among a Medicaid-insured population with SLE using health plan administrative claims.
Methods
DATA SOURCE
This retrospective study used administrative claims data from the Merative MarketScan Multi-State Medicaid Database (Medicaid) from January 1, 2013, through December 31, 2019 (Supplementary Figure 1 (229.9KB, pdf) , available in online article). The Medicaid Database contains the pooled health care experience of more than 20 million Medicaid enrollees from multiple geographically dispersed states, including all IP admissions and services, OP services, and OP prescription drug claims. All patient records are deidentified, and a unique identifier links each patient’s associated medical and pharmacy claims and enrollment information. Because this study used deidentified patient records, pursuant to the Health Insurance Portability and Accountability Act of 1996, institutional review board approval was not required. Study data were captured using International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM/ICD-10-CM) codes, Current Procedural Terminology fourth edition codes, the Healthcare Common Procedure Coding System, and National Drug Code numbers.
STUDY DESIGN
This study identified a cohort of patients with prevalent SLE, specifically a population containing patients at any points in the SLE patient journey, rather than a population of patients with incident SLE. To this end, the index date was randomly selected among SLE service dates that were at least 12 months after the earliest identified SLE diagnosis. Patients were required to have at least 1 IP claim with an SLE diagnosis (ICD-9-CM: 710.0x or ICD-10-CM: M32-) or at least 2 nondiagnostic OP claims (ie, not diagnostic tests or screening) separated by 30-365 days. Patients were required to be at least age 18 years on index date and continuously enrolled for 12 months before the index date (year 1) and 12 months following the index date (year 2) (Supplementary Figure 1 (229.9KB, pdf) ).7 Because the cumulative oral corticosteroid (OCS) dose was considered a potential predictor of flares and an indicator of disease severity, patients were excluded if they had any OCS claims that were determined to be clinically invalid (eg, a prednisone-equivalent dose >200 mg/day or missing/zero value for the day’s supply or quantity). There were no exclusion criteria for other SLE treatments because the dose was not a measured predictor of interest.
STUDY OUTCOMES
The primary study outcome was SLE flares that required an IP admission or ED visit in year 2. Flares that required an IP/ED visit were chosen as the primary outcome because of the burden they place on both patients and health care systems. SLE flares and disease severity were identified using the Garris algorithm, a previously published real-world algorithm validated with administrative claims.8 Flares were then further classified as those that required an IP admission with a primary diagnosis of SLE or a specified SLE-related condition (eg, end-stage renal disease or venous thrombosis) or an ED visit with a primary SLE diagnosis or secondary diagnosis for an SLE-related condition (see Supplementary Table 1 (229.9KB, pdf) for SLE-related conditions).
Study Measures. Patient demographic characteristics were captured on index date and included age, sex, race, insurance plan type, and urbanicity. Comorbid conditions were identified by at least 1 claim with a diagnosis in any position during year 1. These included components of the Elixhauser Comorbidity Index (ECI) score, including cancer, chronic pulmonary disease, coagulopathy, congestive heart failure, deficiency anemia, depression, diabetes, drug abuse, fluid and electrolyte disorders, hypertension, hypothyroidism, liver disease, obesity, neurological disorders (including dementia, seizures, epilepsy, and neurological disorders affecting movement) and other neurological disorders, and peripheral vascular disorders.9,10 Other measured comorbidities included SLE-related comorbidities as the components used in the SLE-specific risk-adjusted index developed by Ward10: anxiety, avascular necrosis, cardiovascular disease, chronic kidney disease, deep vein thrombosis/venous thromboembolic disease, fatigue, fever, fibromyalgia, fractures, glaucoma, headache, kidney transplant, lupus nephritis, osteoarthritis, osteoporosis, pleurisy/pleural effusion, pulmonary embolism, Raynaud disease, seizure, and thrombocytopenia.
SLE medication use in year 1 was measured in 2 ways. First medications were collected as a binary predictor indicating whether a patient had at least 1 claim for each class of medication (antimalarials, immunosuppressants, biologics, and systemic corticosteroids/OCSs). Second, SLE treatment progression was classified hierarchically by identifying the most advanced treatment a patient had received in year 1 (ie, no treatment, any antimalarial use but no immunosuppressant/biologic use, any immunosuppressant use but without biologic use, or any biologic use).3 For example, if a patient had a claim for both an antimalarial medication and a biologic, they would be classified as having biologic use. Use of concomitant medications (antidepressants, antihypertensives, or opioids) were also reported in year 1. Additional measures of SLE disease activity in year 1 included cumulative OCS dosage and the total number of SLE flares in year 1, as defined by the Garris algorithm.8
All-cause and SLE-related health care resource use and costs were evaluated during year 1 as covariates. All-cause visits were defined as any IP or OP visit, regardless of diagnosis or treatment. SLE-related visits were defined as claims with an SLE diagnosis in any position, OP pharmacy claims for SLE treatments, or SLE-related comorbidities from the Ward SLE-specific risk-adjusted index9,10 identified in any position of IP and ED claims. Costs included amounts paid by both health plans and patients for services, including medical and pharmacy costs. All costs were adjusted for inflation using the medical care component of the Consumer Price Index obtained from the US Bureau of Labor Statistics and standardized to 2019 US dollars.
STATISTICAL ANALYSIS
Demographic and baseline clinical characteristics were summarized descriptively. Counts and proportions were used to describe categorical variables, whereas means and SDs were used to describe continuous variables.
To identify factors associated with flares that required an IP/ED visit, 2 modeling approaches were implemented. First, multivariable logistic regression modeling was used to examine the linear relationship between year 1 predictors and the risk of a flare-related IP/ED visit in year 2; all covariates listed throughout the Methods section (demographic and clinical characteristics, SLE medications, SLE flares in year 1, health care resource utilization in year 1) were included in the logistic regression model (for a full list of covariates, see Supplementary Table 2 (229.9KB, pdf) ). Second, a classification and regression tree (CART) model was used to generate a decision tree for predicting the risk of flares-related IP/ED visit based on the combination of predictors.11 CART models select an optimal combination of predictors that can clearly identify subgroups of at-risk populations and, therefore, inform recommendations.12 Additionally, CART models are not subject to the limitation of multicollinearity of a logistic regression model.
The study dataset was randomly split into training (75% of patients) and validation (remaining 25% of patients) datasets with which the CART model was fitted and validated. At each node, the tree was split on the predictor and split value that minimized the Gini impurity. The splitting process continued within each new data partition until the maximum tree depth was achieved. The tree was then pruned to avoid overfitting by penalizing the purity criterion using a complexity parameter, a factor of the total number of terminal nodes in the tree. The optimal complexity parameter was selected using 10-fold cross validation repeated 10 times. The tree’s predictive performance was evaluated in the validation dataset using the area under the receiver operator curve, also known and the C-statistic, and the Brier score.
The predicted probability of having a flare was computed for each patient in the validation dataset. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were computed at various predicted probability cutoffs for classification. Predicted probability at least 0.3 was used to classify patients as predicted to have an IP or ED flare. Lastly, the relative variable importance will be reported for all predictors included in the CART model, with the percent improvement reported for each variable as compared with the most important variable.
Descriptive analyses were conducted using WPS version 4.2 (World Programming), multivariable and CART analyses were conducted using R version 3.6.3, and the “rpart” package.
Results
DEMOGRAPHIC AND YEAR 1 CHARACTERISTICS
After applying patient selection criteria, the study identified 8,083 patients with SLE in the Medicaid database. Most patients were female (93.3%), and the mean (SD) age of patients was 40.9 (12.3) years (Table 1 and Supplementary Table 3 (229.9KB, pdf) ). Overall, 46.9% of patients were Black, 38.3% were White, and 14.7% identified as another race. The most frequently observed year 1 comorbidities included hypertension (52.3%), anxiety (34.8%), depression (34.8%), deficiency anemia (26.1%), fibromyalgia (25.2%), and obesity (24.5%). SLE treatments commonly prescribed in year 1 included systemic corticosteroids (36.0%), antimalarials (55.2%), and immunosuppressants (32.3%); biologics were prescribed to 4.9% of patients. Of those without biologics, 28.8% were prescribed immunosuppressants; and, of the remainder, 30.4% were prescribed only antimalarials. Notably, 51.3% of patients were prescribed an opioid in year 1.
TABLE 1.
Select Demographics and Year 1 Characteristics (Full Table Available in the Supplementary Materials)
| Characteristics and demographics | All patients (N = 8,083) | |
|---|---|---|
| Demographic characteristicsa | ||
| Age, mean SD, y | 40.9 | 12.3 |
| Female sex, n % | 7,537 | 93.3 |
| Race, n % | ||
| White | 3,097 | 38.3 |
| Black | 3,794 | 46.9 |
| Other | 1,192 | 14.7 |
| Urbanicity, urban, n % | 6,312 | 78.1 |
| Clinical characteristicsb | ||
| Selected ECI conditions, n % | ||
| Chronic pulmonary disease | 1,937 | 24.0 |
| Depression | 2,809 | 34.8 |
| Diabetes | 1,375 | 17.0 |
| Hypertension | 4,225 | 52.3 |
| Hypothyroidism | 977 | 12.1 |
| Obesity | 1,982 | 24.5 |
| Other neurological disordersc | 1,387 | 17.2 |
| Renal failure | 840 | 10.4 |
| SLE-related comorbidities, n %d | ||
| Anxiety | 2,816 | 34.8 |
| Atherosclerosis | 546 | 6.8 |
| Cerebrovascular diseasee | 719 | 8.9 |
| Endocarditis | 774 | 9.6 |
| Myocardial infarction | 283 | 3.5 |
| Pericarditis | 206 | 2.5 |
| Chronic kidney disease | 1,093 | 13.5 |
| Fibromyalgia | 2,040 | 25.2 |
| Fractures | 455 | 5.6 |
| Kidney transplant | 50 | 0.6 |
| Lupus nephritis | 1,207 | 14.9 |
| Osteoarthritis | 1,916 | 23.7 |
| Osteoporosis | 440 | 5.4 |
| Pleurisy/pleural effusion | 396 | 4.9 |
| Raynaud disease | 495 | 6.1 |
| Thrombocytopenia | 500 | 6.2 |
| Number of flares, mean SD | 3.9 | 2.0 |
| Treatment characteristicsb | ||
| SLE treatments, n % | ||
| Antimalarials | 4,460 | 55.2 |
| Biologics | 397 | 4.9 |
| Immunosuppressants | 2,609 | 32.3 |
| Systemic corticosteroids | 2,907 | 36.0 |
| Cumulative OCS dosef in 100 mg, mean SD | 9.15 | 17.32 |
| Most advanced SLE treatments, n % | ||
| No antimalarial, immunosuppressant, or biologic use | 2,905 | 35.9 |
| Antimalarial (without immunosuppressant or biologic) | 2,454 | 30.4 |
| Immunosuppressant (without biologic use) | 2,327 | 28.8 |
| Biologic | 397 | 4.9 |
| Concomitant medications, n % | ||
| Antidepressants | 4,220 | 52.2 |
| Antihypertensives | 4,539 | 56.2 |
| Opioids | 4,148 | 51.3 |
| All-cause health care utilization and costsb | ||
| Patients with an IP admission, n % | 2,128 | 26.3 |
| Patients with an ED visit, n % | 5,649 | 69.9 |
| Total health care costs, mean SD, USD | $19,996 | $59,337 |
a Demographic characteristics were measured on the index date.
b Clinical characteristics, treatment characteristics, and health care utilization and costs were measured during the 12-month period before the index date.
c “Other” neurological conditions are defined in the ECI as neurological conditions other than dementia, seizures and epilepsy, or neurological disorders affecting movement.
d The SLE-related comorbidities were adapted from Ward 2000. 10
e Cardiovascular disease is inclusive of cerebrovascular disease and myocardial infarction, as well as other cardiovascular-related conditions.
f OCS dosing was measured in prednisone-equivalent doses.
ECI = Elixhauser Comorbidity Index; ED = emergency department; IP = inpatient; OCS = oral corticosteroid; SLE = systemic lupus erythematosus; USD = United States dollar; y = year.
In year 1, 26.3% of patients had at least 1 all-cause IP admission, and 69.9% had at least 1 all-cause ED visit.13 Average total all-cause health care costs during year 1 were $19,996 (SD = $59,337) (Table 1 and Supplementary Table 3 (229.9KB, pdf) ).
PREDICTORS OF SLE FLARE-RELATED IP/ED VISITS: LOGISTIC REGRESSION MODEL
In year 2, 37.6% of patients (n = 3,039) had an SLE flare that required an IP/ED visit, with 9.1% of patients specifically having at least 1 flare-related IP stay and 34.0% having at least 1 flare-related ED visit (Table 2). Results from adjusted logistic regression models indicate that patients with at least 1 ED visit in year 1 had more than twice the odds of having a flare-related IP/ED visit in year 2 (odds ratio [OR] = 2.19, 95% CI = 1.93-2.49) than those without a year 1 ED visit. Compared with no SLE treatment, SLE treatment with biologics (OR = 0.54, 95% CI = 0.42-0.70), immunosuppressants (OR = 0.59, 95% CI = 0.51-0.68), or antimalarials (OR = 0.54, 95% CI = 0.42-0.70) was associated with decreased odds of a flare-related IP/ED visit in year 2. The other predictors of a flare-related IP/ED visit in year 2 were ECI-defined other neurological disorders (OR = 1.63, 95% CI = 1.43-1.87), Black race (OR = 1.49, 95% CI = 1.32-1.68), and chronic kidney disease/renal failure (OR = 1.35, 95% CI = 1.10-1.66). Of note, total health care costs (per 10% increase) in year 1 were not associated with the odds of a flare-related IP/ED visit in year 2. Full results from the adjusted logistic regression models are reported in Figure 1 (significant factors; P < 0.05) and Supplementary Table 2 (229.9KB, pdf) (full model results).
TABLE 2.
Frequency and Proportion of SLE Flare-Related IP/ED Visit in Year 2
| Health care utilization | All patients (N = 8,083) | |
|---|---|---|
| Patients with an SLE flare-related IP/ED visit, n % | 3,039 | 37.6 |
| Patients with a flare-related IP admission, n % | 738 | 9.1 |
| Patients with a flare-related ED visit, n % | 2,748 | 34.0 |
ED = emergency department; IP = inpatient; SLE = systemic lupus erythematosus.
FIGURE 1.
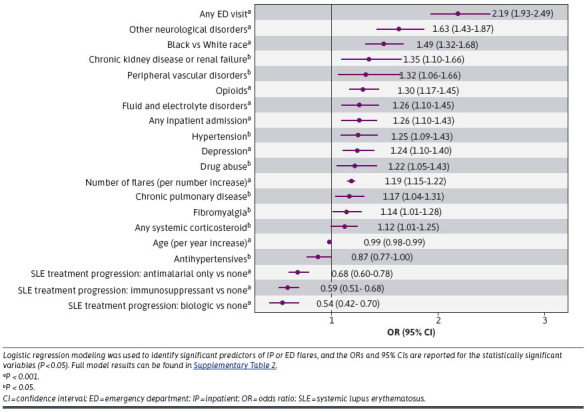
Associations Between Year 1 Patient Characteristics With Any SLE Flare-Related IP/ED Visit in Year 2 Based on Logistic Regression Modeling
PREDICTORS OF SLE FLARE-RELATED IP/ED VISITS: CART MODEL
The variable importance of year 1 characteristics was assessed and reported in Table 3. Variable importance identified opioids (variable importance = 1.00), other neurological disorders (0.96), any ED visit (0.92), any IP admission (0.89), and depression (0.64) as the variables given the strongest weight in CART modeling (Table 3).
TABLE 3.
Variable Importance of Year 1 Covariates Potentially Related to Any Flare-Related Inpatient or ED Visits in Year 2 Based on Classification and Regression Tree Model
| Predictor | Variable importance |
|---|---|
| Opioids | 1.000 |
| Other neurological disordersa | 0.964 |
| Any ED visit | 0.918 |
| Any inpatient admission | 0.892 |
| Depression | 0.643 |
| Chronic kidney disease or renal failure | 0.318 |
| Any systemic corticosteroid | 0.135 |
| Lupus nephritis | 0.099 |
| Race | 0.090 |
| Hypertension | 0.089 |
| Pericarditis | 0.027 |
The following characteristics were also included in the model but had a variable importance of 0.000: aged at least 45 years, aged at least 65 years, sex, insurance plan type, cancer, chronic pulmonary disease, coagulopathy, congestive heart failure, diabetes, drug abuse, liver disease, peripheral vascular disorders, anxiety, atherosclerosis, cerebrovascular disease/stroke/transient ischemic attack, endocarditis, myocardial infarction, fibromyalgia, fractures, kidney transplant, osteoarthritis, osteoporosis, pleurisy/pleural effusion, Raynaud disease, thrombocytopenia, antidepressants, and antihypertensives.
a “Other” neurological disorders are defined in the Elixhauser Comorbidity Index as neurological conditions other than dementia, seizures and epilepsy, or neurological disorders affecting movement.
ED = emergency department.
The CART model identified combinations of attributes useful to determining the risk of year 2 IP or ED SLE flares. Patients with a year 1 ED visit, a year 1 IP admission, and evidence of other neurological disorders had the highest probability of a flare requiring an IP/ED visit in year 2 (probability = 0.708), whereas patients without a year 1 ED visit had the lowest probability of a year 2 flare-related IP/ED visit (probability = 0.185) (Figure 2). The C-statistic for selected covariates was 0.72.
FIGURE 2.
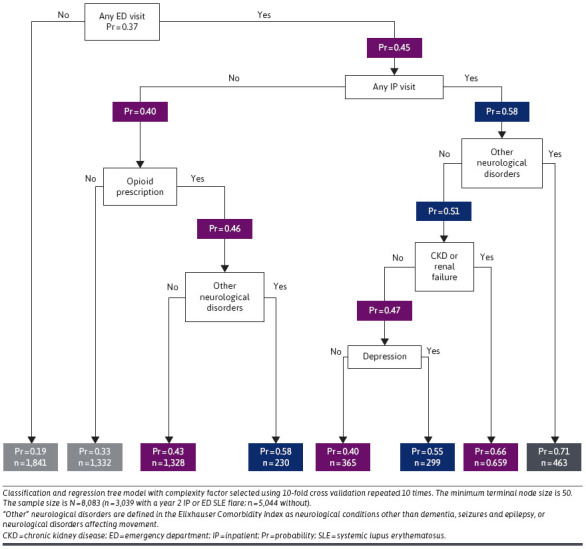
Classification and Regression Tree Model of Associations Between Year 1 Patient Characteristics and Any SLE Flare-Related IP/ED Visit in Year 2
Discussion
The results of this analysis highlight the substantial unmet need for improved management of SLE among Medicaid patients, as demonstrated by the fact that more than one-third of the SLE patients in our study had at least 1 SLE flare-related IP/ED visit during year 2. ED visits during year 1 were found to be an important predictor of year 2 IP or ED SLE flares in both the logistic regression and CART models.
Our results provide data on opioids and acute care use among patients with SLE. In this study, opioid use was identified as the factor with the highest variable importance but was only included in the classification tree for those patients with an ED visit and no IP admission. This supports previous research reporting that pain management is one of the major reasons for ED use among patients with SLE.14 Additionally, more than half of the patients with SLE had evidence of opioid use during year 1. Although it is unclear whether the opioids were prescribed for the treatment of SLE, the high proportion of patients with SLE with evidence of opioid use during year 1 may be indicative of patients with SLE attempting to manage significant pain. Previous work has shown a similarly high use of opioids among patients with SLE, despite opioids not being indicated for the treatment of long-term musculoskeletal pain.15,16 This provides actionable insight by identifying patients with prior opioid use as potential targets for intervention to decrease flare-related IP/ED visits.
Another interesting finding from this study was that mental health, specifically depression (OR = 1.24, 95% CI = 1.10-1.40), was a more important predictor in the logistic regression model than several physical conditions, such as cancer, congestive heart failure, diabetes, obesity, cerebrovascular disease/stroke/transient ischemic attack, kidney transplant, lupus nephritis, and osteoarthritis. Depression was also selected for inclusion by the CART models. Those patients who had an ED visit, an IP visit, another neurological disorder, chronic kidney disease or renal failure, and depression had a 55% probability of having a flare-related IP/ED visit, compared with a 40% probability among those without depression. This highlights the importance of comprehensively managing mental health for patients with SLE.
Among the demographic characteristics of Medicaid-insured patients with SLE, Black race was found to be a significant predictor of IP or ED flares in the logistic regression model. This increased risk of SLE flare-related IP/ED visits among Black patients is consistent with prior literature showing that Black patients are not only more likely to develop SLE flares but also to have a higher risk of more severe disease, including irreversible organ damage, end-stage renal disease, and death.17-22 Improving disease management among Black patients, including reducing SLE flares, is critical to reducing the overall and disproportionate disease burden. However, despite the increased risk observed in the logistic regression model, Black race did not have high variable importance in the CART model (variable importance = 0.090) and was not included in the predictive tree. Previous research has shown that non-White patients with SLE have less access to primary care,23 which, combined with the variable importance, may suggest that race is acting as a proxy for more critical health care use (eg, ED visits and IP admissions) and is an area on which future research could be focused. Therefore, SLE outcomes for Black patients with SLE could be improved through greater access to primary care as well as providers identifying those patients most at risk based on the CART model rather than relying broadly on race as a risk factor for IP or ED flares.
This study also highlights the potential to reduce flare-related IP/ED visits through improved pharmacological treatment. In the logistic regression model, more advanced pharmacological treatment in year 1 was associated with a decreased risk of a flare-related IP/ED visit in year 2. Biologic treatment compared with no treatment reduced flare risk by 46% compared with 41% for immunosuppressants and 32% for antimalarials. This suggests a positive association of IP or ED flare reduction with the use of advanced pharmacologic treatment for SLE. With the information gained from this study, future research should focus on medication adherence and discontinuation in relation to the timing of the SLE flare.24,25
LIMITATIONS
There are several limitations associated with the results of this study. Clinical characteristics and identification of SLE flares were ascertained based on diagnosis codes, procedure codes, and pharmacy prescriptions in claims, which are subject to data coding limitations and data entry error; this may have resulted in an underestimation of flares because of a lack of information on biomarkers previously used to identify SLE flares. Similarly, as treatment outcomes are based on claims, we assume that patients took medications as prescribed; there was no confirmation through chart review or patient contact confirming that patients took the medications. Second, results from this study are limited to patients with SLE receiving Medicaid coverage in select states and, therefore, are not representative of all patients with Medicaid or SLE. Results may not be generalizable to patients with SLE with other insurance types or without health insurance coverage. Additionally, because patients were required to have 2 years of continuous enrollment, there may be survivorship bias because patients who had more severe disease and died during the study period were not eligible for inclusion. Finally, the logistic regression and CART models were limited to predictors that are reported in administrative claims, which does not include social determinants of health, such as income and access to care, as well as other predictors of flares (eg, laboratory values).
Conclusions
This study examined a broad array of patient-level demographic, clinical, and pharmacological risk factors, and identified the most important predictors of flare-related IP/ED visits using 2 predictive methodologies. From the CART model, patients with a prior ED visit, IP admission, and a diagnosis for an ECI-defined other neurological disorder had the highest probability (70.8%) of a flare-related IP/ED visit and represent a population that would benefit from targeted intervention. Additionally, in logistic regression models, patients with SLE with prior opioid use and Black patients with SLE were identified as specific subgroups with a high risk of a flare-related IP/ED visit, indicating that these groups need improved disease management. Finally, more advanced pharmacological SLE treatments, particularly biologic therapy, was associated with a lower likelihood of a flare-related IP/ED visit, highlighting a treatment effect for effective management of SLE and flare reduction.
ACKNOWLEDGMENTS
The authors thank Joseph Tkacz for contributing to the study cohorts’ creation and Helen Varker and Richard Bizier of Merative (formerly IBM Watson Health at the time of analysis) for supporting programming services of this study. The authors also wish to acknowledge Megan Richards and Liisa Palmer of Merative for their technical editing of the manuscript.
Funding Statement
This study was funded by AstraZeneca.
REFERENCES
- 1.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Manifestations of systemic lupus erythematosus. Maedica (Bucur). 2011;6(4):330-6. [PMC free article] [PubMed] [Google Scholar]
- 2.Stojan G, Petri M. Epidemiology of systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2018;30(2):144-50. doi:10.1097/BOR.0000000000000480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fanouriakis A, Kostopoulou M, Alunno A, et al. . 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736-45. doi:10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- 4.García-Carrasco M, Mendoza Pinto C, Solís Poblano JC, Etchegaray Morales I, Cervera R, Anaya JM. Systemic lupus erythematosus. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al. , eds. Autoimmunity: From Bench to Bedside. El Rosario University Press; 2013. [PubMed] [Google Scholar]
- 5.Thanou A, Jupe E, Purushothaman M, Niewold TB, Munroe ME. Clinical disease activity and flare in SLE: Current concepts and novel biomarkers. J Autoimmun. 2021;119:102615. doi:10.1016/j.jaut.2021.102615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R, Guthridge JM, Chen H, et al. . Immunologic findings precede rapid lupus flare after transient steroid therapy. Sci Rep. 2019;9(1):8590. doi:10.1038/s41598-019-45135-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu SS, Perry A, Tkacz J, Bryant G. Clinical and economic characterization of mild, moderate, and severe systemic lupus erythematosus: Real-world observation across payer channels in the United States. J Manag Care Spec Pharm. 2023;29(9):1010-20. doi:10.18553/jmcp.2023.29.9.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garris C, Jhingran P, Bass D, Engel-Nitz NM, Riedel A, Dennis G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ. 2013;16(5):667-77. doi:10.3111/13696998.2013.778270 [DOI] [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 10.Ward MM. Development and testing of a systemic lupus-specific risk adjustment index for in-hospital mortality. J Rheumatol. 2000;27(6):1408-13. [PubMed] [Google Scholar]
- 11.Speybroeck N. Classification and regression trees. Int J Public Health. 2012;57(1):243-6. doi:10.1007/s00038-011-0315-z [DOI] [PubMed] [Google Scholar]
- 12.Krzywinski M, Altman N. Classification and regression trees. Nat Methods. 2017;14(8):757-8. doi:10.1038/nmeth.4370 [Google Scholar]
- 13.Tkacz J, Perry A, Varker H, Bizier R, Ortmann R. Sze-jung Wu S. Clinical and economic characterization of systemic lupus erythematosus patients: Real world observation across disease severity and payer channels in the U.S. Abstract presented at: ACR Convergence 2021; November 6, 2021. [Google Scholar]
- 14.Lee J, Lin J, Suter LG, Fraenkel L. Persistently frequent emergency department utilization among persons with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2019;71(11):1410-8. doi:10.1002/acr.23777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birt JA, Wu J, Griffing K, et al. . Corticosteroid dosing and opioid use are high in patients with SLE and remain elevated after belimumab initiation: a retrospective claims database analysis. Lupus Sci Med. 2020;7(1):e000435. doi:10.1136/lupus-2020-000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somers EC, Lee J, Hassett AL, et al. . Prescription opioid use in patients with and without systemic lupus erythematosus - Michigan Lupus Epidemiology and Surveillance Program, 2014-2015. MMWR Morb Mortal Wkly Rep. 2019;68(38):819-24. doi:10.15585/mmwr.mm6838a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petri M, Purvey S, Fang H, Magder LS. Predictors of organ damage in systemic lupus erythematosus: The Hopkins Lupus Cohort. Arthritis Rheum. 2012;64(12):4021-8. doi:10.1002/art.34672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González LA, Toloza SM, McGwin G Jr, Alarcón GS. Ethnicity in systemic lupus erythematosus (SLE): its influence on susceptibility and outcomes. Lupus. 2013;22(12):1214-24. doi:10.1177/0961203313502571 [DOI] [PubMed] [Google Scholar]
- 19.Ugarte-Gil MF, Acevedo-Vásquez E, Alarcón GS, et al. ; GLADEL . The number of flares patients experience impacts on damage accrual in systemic lupus erythematosus: Data from a multiethnic Latin American cohort. Ann Rheum Dis. 2015;74(6):1019-23. doi:10.1136/annrheumdis-2013-204620 [DOI] [PubMed] [Google Scholar]
- 20.Bruce IN, O’Keeffe AG, Farewell V, et al. . Factors associated with damage accrual in patients with systemic lupus erythematosus: Results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74(9):1706-13. doi:10.1136/annrheumdis-2013-205171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alarcón GS, McGwin G Jr, Petri M, et al. ; PROFILE Study Group . Time to renal disease and end-stage renal disease in PROFILE: A multiethnic lupus cohort. PLoS Med. 2006;3(10):e396. doi:10.1371/journal.pmed.0030396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.From the Centers for Disease Control and Prevention. Trends in deaths from systemic lupus erythematosus–United States, 1979-1998. JAMA. 2002;287(20):2649-50. [PubMed] [Google Scholar]
- 23.Brown EA, Gebregziabher M, Kamen DL, White BM, Williams EM. Examining racial differences in access to primary care for people living with lupus: Use of ambulatory care sensitive conditions to measure access. Ethn Dis. 2020;30(4):611-20. doi:10.18865/ed.30.4.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida-Brasil CC, Hanly JG, Urowitz M, et al. . Flares after hydroxychloroquine reduction or discontinuation: Results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann Rheum Dis. 2022;81(3):370-8. doi:10.1136/annrheumdis-2021-221295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji L, Gao D, Hao Y, et al. . Low-dose glucocorticoids withdrawn in systemic lupus erythematosus: a desirable and attainable goal. Rheumatology (Oxford). 2022;62(1):181-9. doi:10.1093/rheumatology/keac225 [DOI] [PubMed] [Google Scholar]


