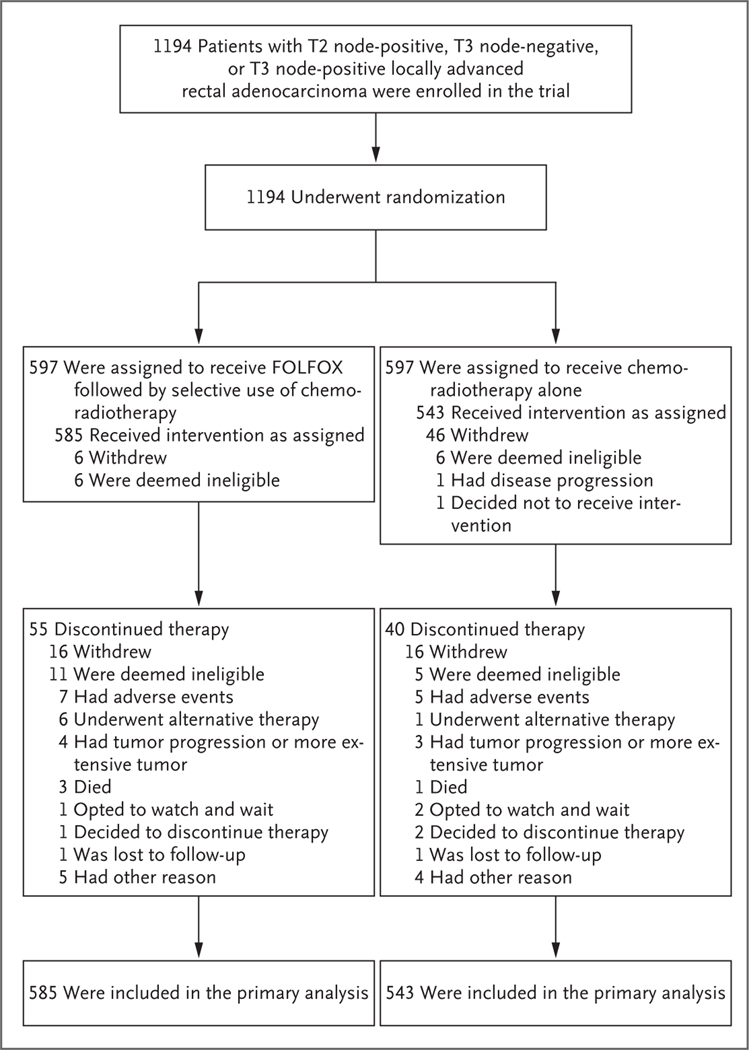
Figure 1. Enrollment, Randomization, and Follow-up.
Sites were not required to provide screening logs during the recruitment phase, and therefore the number of patients assessed for eligibility is not available. FOLFOX consists of fluorouracil, leucovorin, and oxaliplatin; the chemoradiotherapy used in the trial consisted of pelvic radiation therapy plus sensitizing chemotherapy with a fluoropyrimidine. Patients in the FOLFOX group received six cycles of FOLFOX, with chemoradiotherapy given only if the primary tumor decreased in size by less than 20% or if FOLFOX was discontinued because of side effects; patients in the chemoradiotherapy group received chemoradiotherapy alone.