Scheme 1.
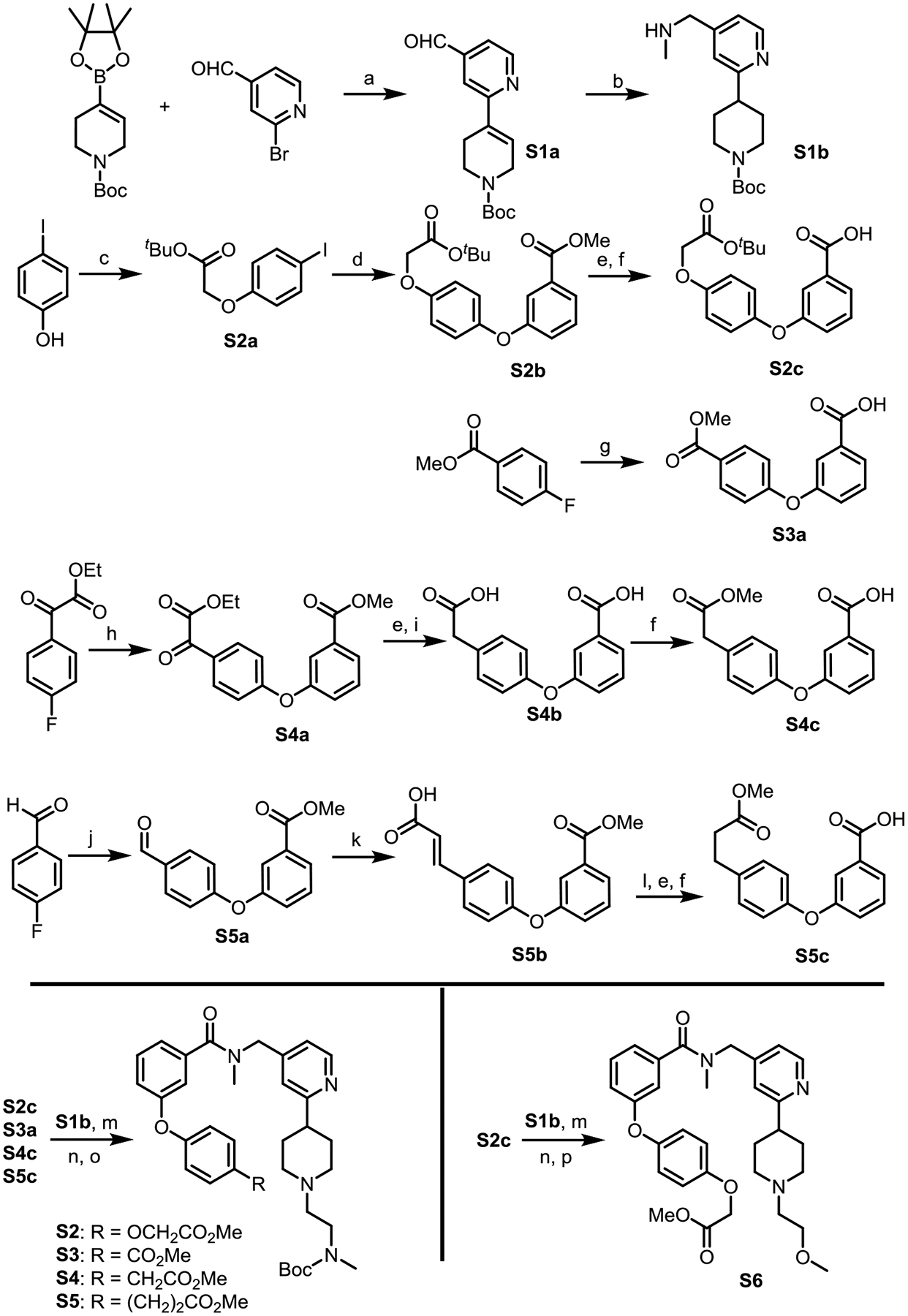
Synthesis of the functionalized CARM1 binder
Reagents and conditions: (a) Pd(dppf)Cl2, Na2CO3, DME/H2O (5/1), 80 °C, yield 59%; (b) MeNH2, Pd/C, H2 (1.0 atm), MeOH, rt; (c) tert-Butyl bromoacetate, K2CO3, KI, Acetone, 60 °C; (d) Methyl 3-hydroxybenzoic acid, CuI, dimethylglycine, Cs2CO3, dioxane, 90 °C, yield 56%; (e) NaOH, THF/MeOH/H2O, rt; (f) Amberlyst-15, MeOH, rt; (g) 3-Hydroxybenzoic acid, NaH (2.1 equiv), DMSO, 70 °C; (h) K2CO3 (2.1 equiv), DMSO, 70 °C; (i) Hydrazine hydrate, NaOH, Ethylene glycol, 160 °C; (j) Methyl 3-hydroxybenzoate, K2CO3 (2.1 equiv), DMSO, 130 °C; (k) Malonic acid, piperidine (10%), pyridine, reflux; (l) Pd/C, H2 (1.0 atm), MeOH, rt; (m) HATU, DIPEA, DMF, rt; (n) TFA/DCM, rt; (o) N-Boc-(methylamino)acetaldehyde, Pd/C, H2 (1.0 atm), MeOH, rt; (p) 2-Bromoethyl methyl ether, Cs2CO3, acetonitrile, rt.
