Abstract
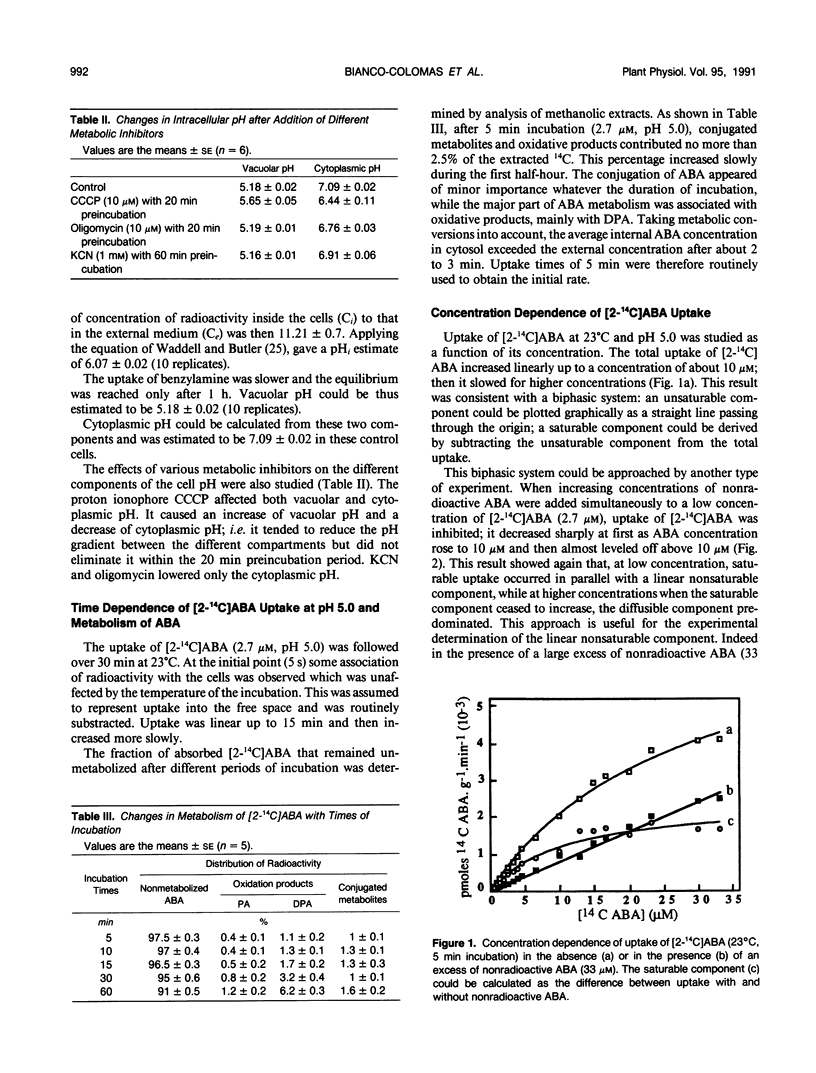
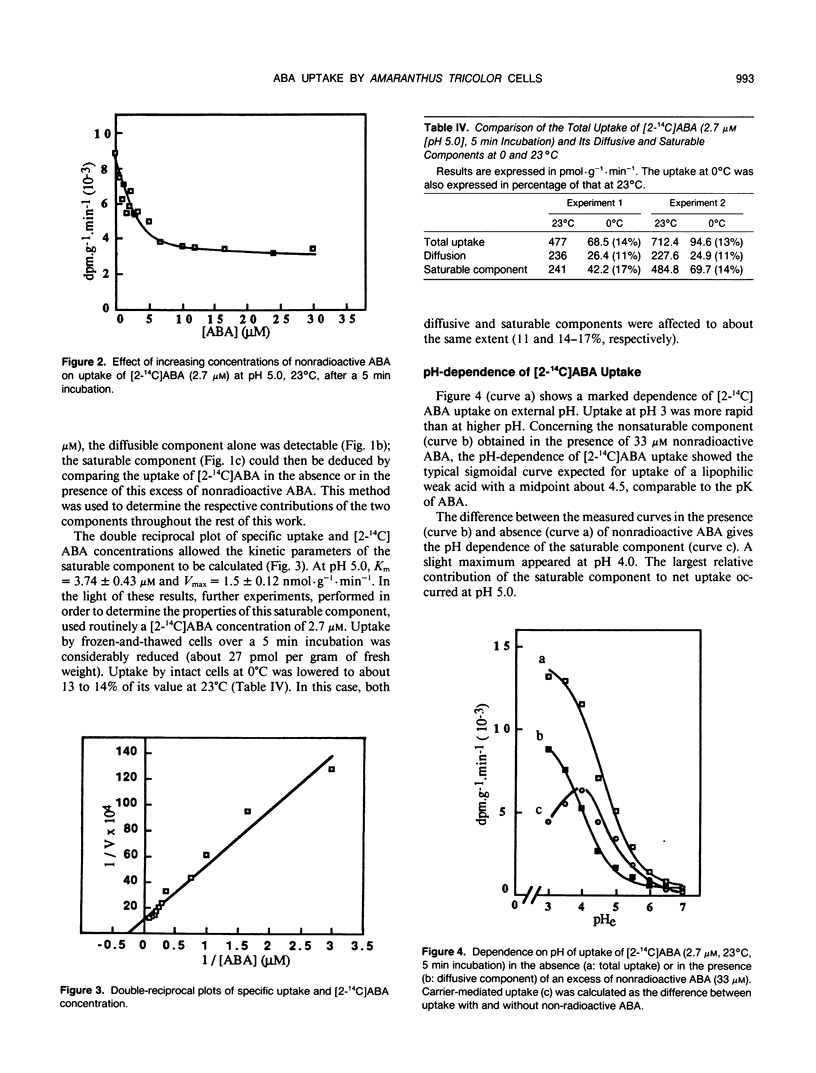
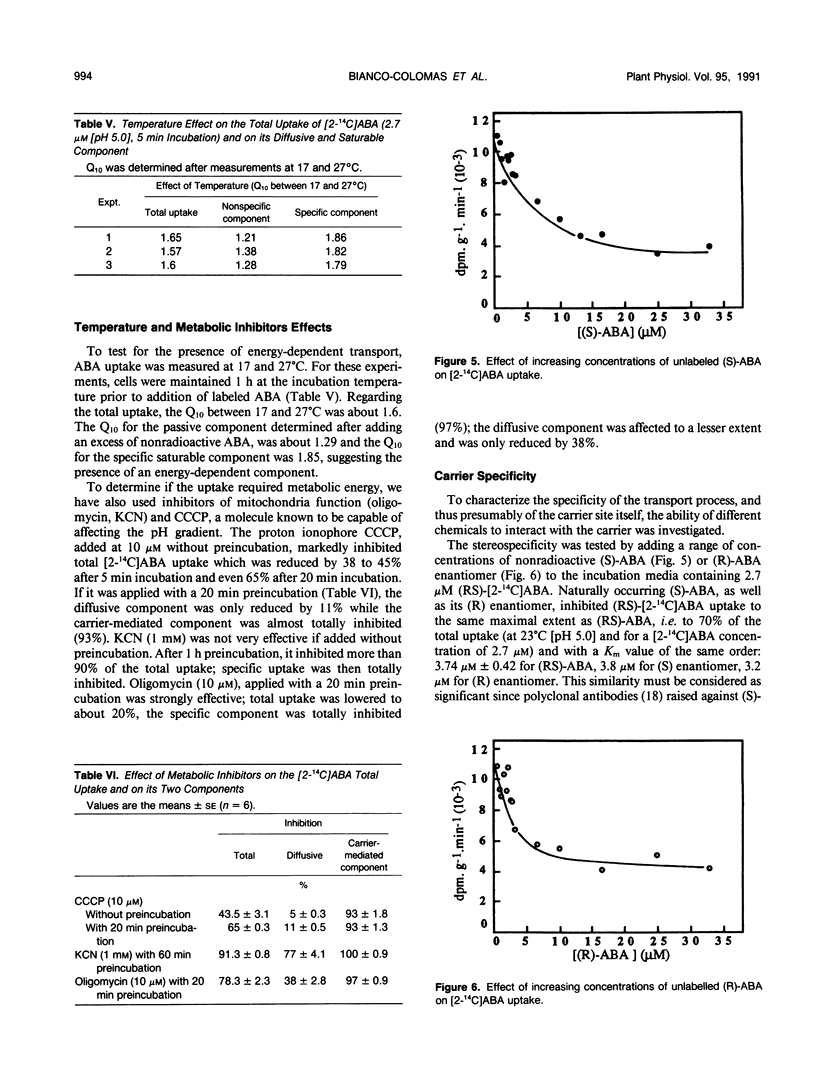
Abscisic acid (ABA) uptake by Amaranthus tricolor cell suspensions was found to include both a nonsaturable component and a saturable part with Km of 3.74 ± 0.43 micromolar and an apparent Vmax of 1.5 ± 0.12 nanomoles per gram per minute. These kinetic parameters as well as the uptake by intact cells at 0°C or by frozen and thawed cells, are consistent with operation of a saturable carrier. This carrier-mediated ABA uptake was partially energized by ΔpH: it increased as the external pH was lowered to pH 4.0; it decreased after the lowering of the ΔpH by the proton ionophore carbonylcyanide-m-chlorophenylhydrazone or after the altering of metabolically maintained pH gradient by metabolic inhibitors (KCN, oligomycin). The carrier is specific for ABA among the plant growth regulators tested, is unaffected by (RS)-trans-ABA and was inhibited by (S)-ABA, (R)-ABA, and also by the ABA analog LAB 173711.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthe P., Bulard C. Anaerobiosis and Release from Dormancy in Apple Embryos: Leaching of (+/-) [C]Abscisic Acid and its Metabolites under Aerobic and Anaerobic Conditions. Plant Physiol. 1983 Aug;72(4):1005–1010. doi: 10.1104/pp.72.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daie J., Wyse R. ABA Uptake in Source and Sink Tissues of Sugar Beet. Plant Physiol. 1983 Jun;72(2):430–433. doi: 10.1104/pp.72.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J., Mathieu Y., Kurkdjian A., Manigault P., Manigault J., Gillet B., Beloeil J. C., Lallemand J. Y. Regulation of Vacuolar pH of Plant Cells: II. A P NMR Study of the Modifications of Vacuolar pH in Isolated Vacuoles Induced by Proton Pumping and Cation/H Exchanges. Plant Physiol. 1989 Jan;89(1):27–36. doi: 10.1104/pp.89.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Hartung W. Uptake and Release of Abscisic Acid by Isolated Photoautotrophic Mesophyll Cells, Depending on pH Gradients. Plant Physiol. 1981 Jul;68(1):202–206. doi: 10.1104/pp.68.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian A., Guern J. Vacuolar pH Measurement in Higher Plant Cells : I. EVALUATION OF THE METHYLAMINE METHOD. Plant Physiol. 1981 May;67(5):953–957. doi: 10.1104/pp.67.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. V., Pilet P. E. Effect of pH on IAA Uptake by Maize Root Segments. Plant Physiol. 1987 Feb;83(2):262–264. doi: 10.1104/pp.83.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., García-Martínez J. L., Adams D., Rappaport L. Uptake and subcellular compartmentation of gibberellin a(1) applied to leaves of barley and cowpea. Plant Physiol. 1980 Sep;66(3):422–427. doi: 10.1104/pp.66.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]


